The NC is a common problem in the paediatric population with an overall incidence of NC being approximately one in 2,500 live births [1]. Aetiology of NC is varied and could be extra hepatic or intra hepatic [2]. However, the most common cause of NC is BA which is surgically correctable [3-5]. The diagnosis of BA and distinguishing it from other causes of NC, specially NH is challenging but necessary [6]. The diagnosis is challenging because there is a high degree of overlap in the clinical, biochemical, imaging, and even histological characteristics of BA and NH [7].
Clinical features of BA and NH are often overlapping and include jaundice, dark urine, acholic stools and hepatosplenomegaly [8]. Ultrasonogram is helpful in detecting absence of gallbladder, which may suggest a diagnosis of BA. HIDA scan is a time consuming and invasive procedure [9]. Intraoperative Cholangiography (IOC) is also an invasive procedure and is performed when the other diagnostic methods do not offer any definitive diagnosis [10]. Although challenging liver biopsy is one of the most reliable methods to differentiate NH from BA [11,12].
There are multiple scoring systems helpful to differentiate BA from NH. One of the scoring systems developed by Gupta DK et al., included only clinical features [13]. Another scoring system devised by the EI-Guindi MAS et al., integrated clinical, radiological, biochemical and histopathological parameters [14]. However, multiple parameters used made it complicated and untenable because often complete clinical work up details were not available for all patients.
Lee WS and Looi LM assigned a scoring system based solely on seven histological parameters for interpretation of liver biopsy in NC [3]. This study was undertaken to apply and validate whether this scoring system could reliably differentiate BA from NH.
Materials and Methods
This was a retrospective descriptive study conducted in the Department of Pathology, Paediatrics and Paediatrics Surgery, in a tertiary care hospital in southern India from January 2010 to June 2014. Ethical clearance was obtained from Institute Ethics committee (Project No.IEC/SC/2012/5/182). Convenient sampling was done based on the number of samples that were received during the study period. All neonates with clinical features of jaundice persisting for more than two weeks in a term infant and more than three weeks in a preterm infant in whom liver biopsy (wedge/core biopsy) was done for NC were included in this study. Liver biopsies done for other hepatobiliary disorders and with inadequate tissue were excluded from this study.
The 4 μm sections were cut and slides were stained with Haematoxylin and Eosin, Reticulin, and Masson Trichrome. Immunohistochemistry was done for Cytokeratin 7 (Leica), Cytokeratin 19 (BioGenex). The slides were studied in detail by the pathologists who were initially blinded to clinicoradiological and biochemical observations. Histopathological scoring was done for the all the cases according to the scoring system devised by the Lee WS and Looi LM highlighted in [Table/Fig-1] and cases were categorised as BA or NH [3]. In all the cases, clinical, biochemical and imaging details were collected and correlated with final diagnosis. Peroperative cholangiogram was taken as the gold standard.
Seven- features, 15- point histological scoring system for the interpretation of liver biopsy in neonatal cholestasis devised by Lee WS and Looi LM [3].
| Parameter | Histological characterization | Histological grade |
|---|
| Portal ductal proliferation | None | 0 |
| Mild | 1 |
| Moderate | 2 |
| Marked | 3 |
| Bile plugin portal ductules | Absent | 0 |
| Present | 2 |
| Porto-portal bridging | None | 0 |
| <50 % of portal tracts | 1 |
| >50% of portal tracts | 2 |
| Lymphocytic infiltrate in portal region | None | 2 |
| Mild | 1 |
| Moderate / severe | 0 |
| Multinucleated hepatocytes | None | 2 |
| Only around central vein | 1 |
| Diffuse | 0 |
| Neutrophils in the infiltrate | Absent or mild | 1 |
| Moderate or marked | 0 |
| Hepatocellular swelling | None | 2 |
| Mild/ focal | 1 |
| Periportal/ diffuse | 0 |
Statistical Analysis
Statistical analysis was done using SPSS software version 20.0. Categorical data was presented as frequencies and percentages. Numerical data was presented as median. Chi-square test and Mann-Whitney U test were used for comparison between the BA and NH patients. Differences were considered as significant at p-value of <0.05. Sensitivity, specificity and predictive value of the scoring system, HIDA scan, POC and histological features were also calculated.
Results
A total of 64 liver biopsies specimens, were received from 51 patients, 44 were obtained via the percutaneous route (core biopsy) and 20 obtained surgically (wedge biopsy). Thirteen patients had both the percutaneous and surgical liver biopsies.
Clinical and Biochemical Features
The clinical, biochemical and imaging details are tabulated in [Table/Fig-2]. Among the 51 patients, 34 were males and 17 were females. Based on histopathological, laboratory, imaging and clinical parameters 25 were diagnosed as BA and 26 as NH. Gender distribution was almost equal for BA (M:F ratio- 1:1.1) patients while NH (M:F ratio-5.5:1) occurred more often in males. One patient with BA had positive anti-CMV IgM antibody while among the 26 NH patients, four had anti-CMV IgM antibody.
Clinical, biochemical and ultrasonographic details of Neonatal cholestasis.
| Findings | Final diagnosis | Totaln=51 | p-value |
|---|
| BA n=25 | NH n=26 |
|---|
| Age at jaundice (In days) Median (Range) | 3(1-120) | 14(1-80) | 14(1-120) | 0.37 |
| Age at biopsy (In days) Median(Range) | 75(32-360) | 48(30-210) | 60(30-360) | 0.001 |
| Male: Female(n) | 12:13 | 22:4 | 34:17 | 0.01 |
| Pale colour StoolNo of the patients(%) | 24(96%) | 13(50%) | 37(72%) | 0.001 |
| Total bilirubin mg/dlMedian(Range) | 11(5.1-33) | 12.5(8.9-24) | 12(5.1-33) | 0.11 |
| Direct bilirubin mg/dL Median(Range) | 4.9(1.3-13.9) | 4.3(1.5-16) | 4.4(1.3-16) | 0.93 |
| AST IU/L Median(Range) | 134(20-3165) | 229(38-1008) | 167(20-3165) | 0.01 |
| ALT IU/L Median(Range) | 65(10-995) | 132(28-879) | 97(10-995) | 0.01 |
| GGT U/L Median(Range) | 386(2.4-980) | 76(22-350) | 103(2.4-980) | 0.001 |
| Atretic gallbladder in USG No of patients(%) | 19(76%) | 7(27%) | 26(51%) | 0.001 |
AST-Aspartate aminotransferase; ALT-Alanine aminotransferase; GGT-Gamma-glutamyl transferase
Histopathological Features
For statistical purpose, moderate and marked bile ductular proliferation were taken together and none and mild bile ductular proliferation were taken together. Four of the seven histological parameters [Table/Fig-1] studied, i.e., moderate to marked bile ductular proliferation (highlighted by Cytokeratin 7 and Cytokeratin 19) was seen in 96%, bile plug was seen in 52%, portal expansion with portoportal bridging involving more than 50% of the portal tracts (highlighted by Reticulin, and Masson Trichrome stain) was seen in 76% and moderate/severe lymphocytic infiltration was seen in 60% of BA cases [Table/Fig-3]. Moderate to marked bile ductular proliferation had the highest (96%) sensitivity in the differentiation of BA from the NH. Both, bile plugs in the bile ductules and portal expansion of more than 50% of the portal tracts had high and equal specificity (88%) [Table/Fig-4]. Diffuse giant cell transformation was seen in 73% and diffuse hepatocytic swelling was seen in 85% of NH cases [Table/Fig-5,6]. Diffuse hepatocytic swelling had higher (85%) sensitivity and diffuse giant cell transformation had 100% specificity for NH [Table/Fig-7].
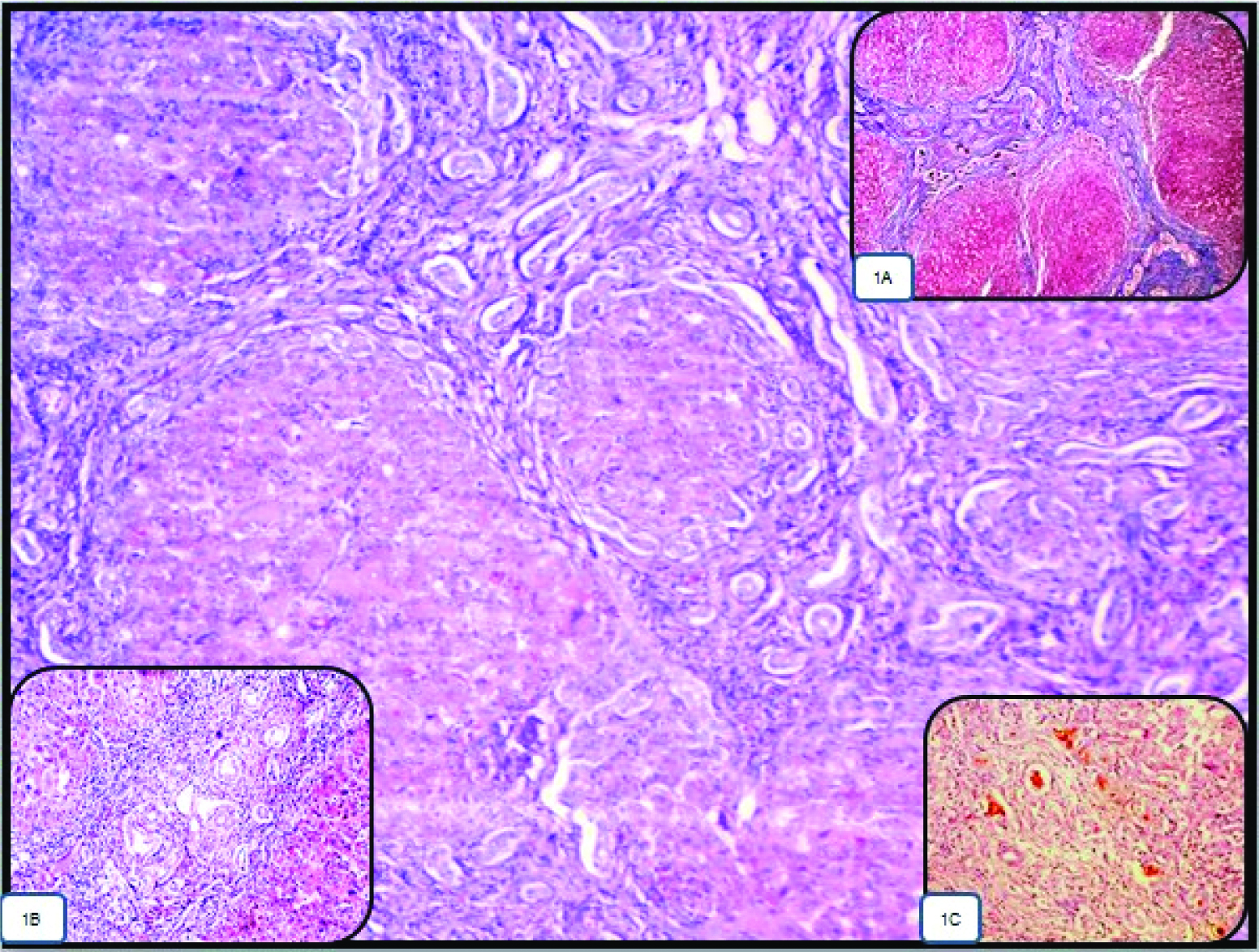
Case of biliary atresia showing portal expansion with portoportal bridging involving the more than 50% of the portal tracts (H&E, 10X). Inset, (1A) highlight the portal fibrosis in the MT stain (MT, 10X), (1B) showing moderate lymphocytic infiltration in the portal tract (H&E, 4X), (1C) showing bile plug in the bile ductules (H&E, 4X).
Sensitivity, specificity, positive predictive value and the negative predictive value of the histopathological features in the differentiation of BA from the NH patients.
| Histological features | BAn=25 | NHn=26 | Sensitivity for BA(%) | Specificity forBA(%) | PPV forBA(%) | NPV forBA(%) |
|---|
| Bile ductular proliferation | Moderate/marked | 24 | 10 | 96 | 62 | 71 | 94 |
| None or mild | 1 | 16 |
| Bile plug in bile ductule | Present | 13 | 3 | 52 | 88 | 81 | 66 |
| Absent/ | 12 | 23 |
| Porto-portal bridging | >50% of portal tracts | 19 | 3 | 76 | 88 | 86 | 79 |
| None/< 50% | 6 | 23 |
| Lymphocytic infiltration | Moderate/severe | 15 | 12 | 60 | 54 | 56 | 58 |
| Absent/mild | 10 | 14 |
| Neutrophilic infiltration | Moderate/severe | 5 | 4 | 20 | 85 | 56 | 52 |
| Absent/mild | 20 | 22 |
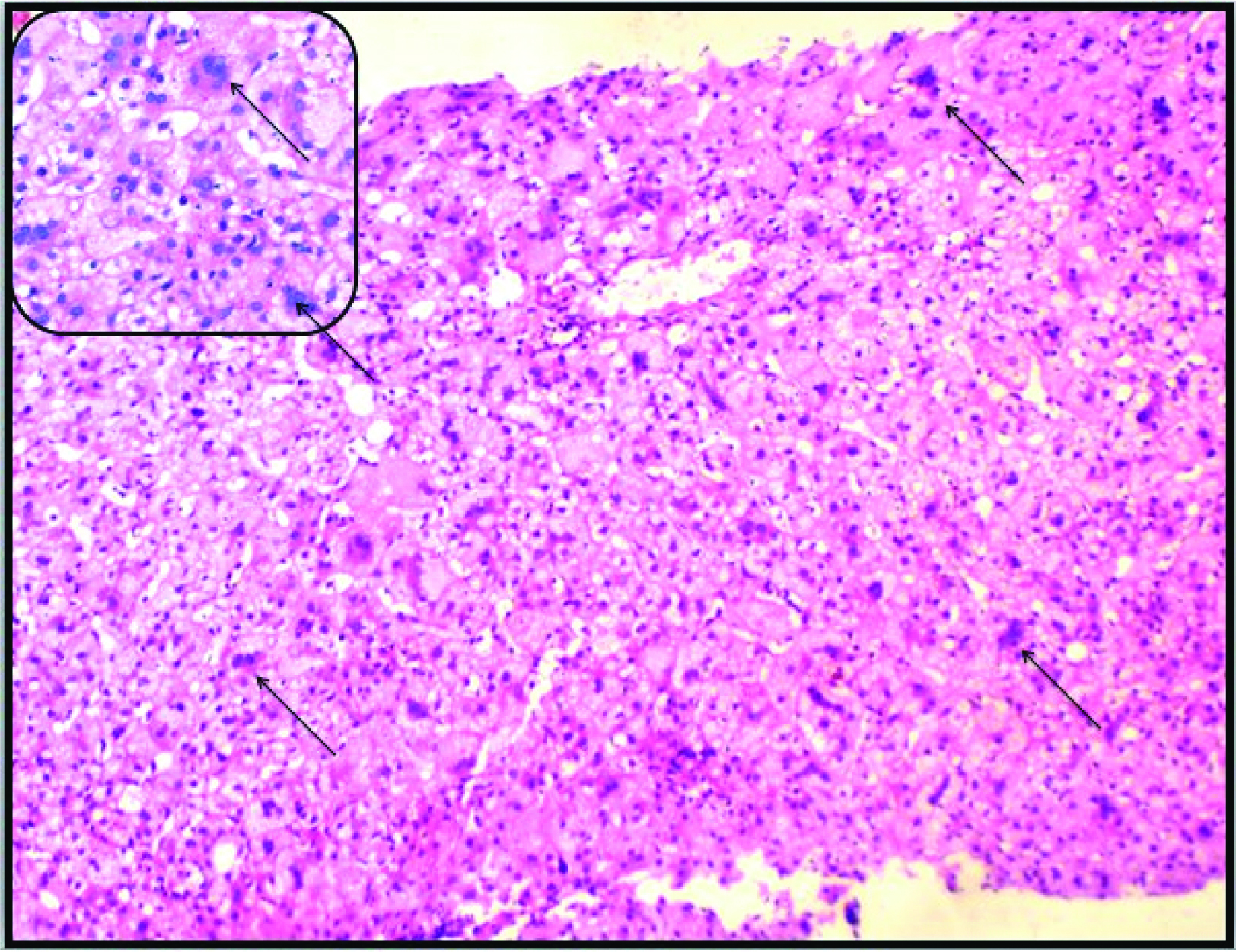
Case of neonatal hepatitis showing diffuse giant cell transformation (arrow) (H&E, 10X). Inset showing the same features in high power (arrow) (H&E, 40X).
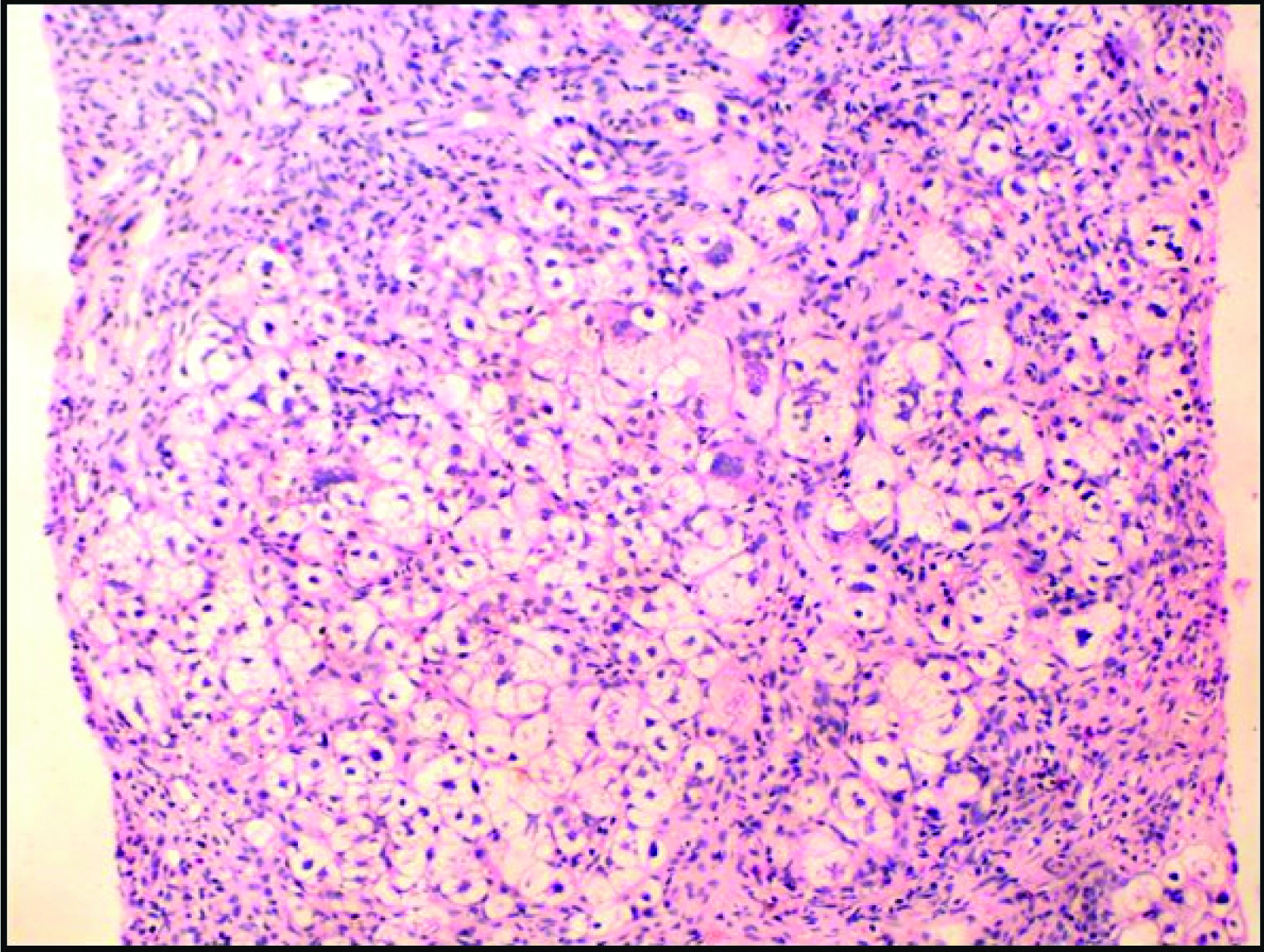
Case of neonatal hepatitis showing diffuse hepatocytes swelling (H&E, 40X).
Sensitivity, specificity, positive predictive value and the negative predictive value of the histopathological features in the differentiation of NH from the BA patients.
| Histological features | NHn=26 | BAn=25 | Sensitivity forNH(%) | Specificity forNH(%) | PPV forNH(%) | NPV forNH(%) |
|---|
| Lymphocytic infiltration | Moderate/severe | 12 | 15 | 46 | 40 | 44 | 42 |
| Absent/mild | 14 | 10 |
| Neutrophilic infiltration | Moderate/severe | 4 | 5 | 15 | 80 | 44 | 48 |
| Absent/mild | 22 | 20 |
| Giant cell transformation | Diffuse | 19 | 0 | 73 | 100 | 100 | 78 |
| None/scattered | 7 | 25 |
| Hepatocytes swelling | Diffuse | 22 | 1 | 85 | 96 | 96 | 86 |
| None/Focal | 4 | 24 |
Comparison of the Histopathological Score with Imaging Studies in BA
The median histopathological score for BA was 10 and for NH, it was four. Among the 25 patients with BA, 24 patients had a score of 7 or more [Table/Fig-8]. A 23 patients including the one with a <7 score, underwent HIDA scan. Twenty-two of them showed non-excretion of the dye into the intestine favouring a diagnosis of BA. The remaining patient although showed excretion of the dye on HIDA scan, showed non- excretion of the dye in POC confirming it to be BA. The two patients who did not undergo HIDA scan, underwent POC directly. In both non excretion of the dye was demonstrated confirming them to be BA also [Table/Fig-9].
Sensitivity, specificity, positive predictive value and the negative predictive value of the scoring system in the differentiation of BA from the NH.
| Histopathological Score | BAn=25 | NHn=26 | Sensitivity for BA (%) | Specificity for BA (%) | PPV for BA (%) | NPV for BA (%) | p-value |
|---|
| ≥7 | 24 | 2 | 92 | 96 | 96 | 92 | 0.001 |
| <7 | 1 | 24 |
Investigation details of the patients diagnosed as BA.
| Final diagnosis | HIDA SCAN | POC |
|---|
| Result | No of the patients | Result | No of the patients |
|---|
| BAn =25 | Non excretion of dye | 22 | Non-excretion of the Dye | 11 |
| Not done | 11 |
| Excretion of the dye | 1 | Non excretion of the dye | 1 |
| Not done | 2 | Non excretion of the dye | 2 |
HIDA scan-Hepatobiliary scintigraphy; POC-Peroperative cholangiogram
Comparison of the Histopathological Score with Imaging Studies in NH
Among the 26 cases of NH, 24 had score less than 7 [Table/Fig-8]. Twenty four of these 26 patients underwent HIDA scan, of whom 16 showed excretion of the dye. Of the remaining eight cases with a non secretory HIDA scan, four underwent POC, in all of whom the dye was excreted. In the remaining four patients, POC was not done. But none of the four patients had pale stools, elevated Gamma glutamyl transferase value, atretic gall bladder and histopathological features which would favour the diagnosis BA so, final diagnosis of NH was considered in these patients. Two patients who did not undergo HIDA scan, showed the excretion of dye on POC [Table/Fig-10].
Investigation details of the patients diagnosed as NH.
| Finaldiagnosis | HIDA SCAN | POC |
|---|
| Result | No of the patients | Result | No of the patients |
|---|
| NHn=26 | Non-excretion of Dye | 8 | Excretion of the Dye | 4 |
| Not done | 4 |
| Excretion of the dye | 16 | Not done | 16 |
| Not done | 2 | Excretion of dye | 2 |
HIDA scan-Hepatobiliary scintigraphy; POC-Peroperative cholangiogram
So overall, in three of the total 51 patients (one in BA and two in NH) there was a discrepancy between the score and the final diagnosis. Imaging studies helped to resolve the issue. When correlated with a score of seven, the scoring system had 92% sensitivity, 96% specificity and 96% positive predictive value in differentiating BA from NH [Table/Fig-8]. Non excretion of dye into the intestine in HIDA scan had 91% sensitivity, 65% specificity, and 73% positive predictive value when correlated with a score of seven or more which favoured the diagnosis of BA.
Discussion
NC is a challenging clinical problem among paediatricians, which needs timely and accurate diagnosis. Overall, NC occurs more often in male patients [7,15]. In our study also, most of the patients were males. Dehghani SM et al., found that age at onset of jaundice for BA was earlier compared to the NH [11]. In our study, although BA presented with jaundice earlier, the patients underwent liver biopsies later than in NH. Generally, NH patients had more severe clinical presentations compared to BA. So usually they came to hospital earlier than BA. A study conducted by Rastogi A et al., and Lee WS and Chai PF also found similar results [7,16]. In contrast, Lee WS and Looi LM, did not find any difference in the age of the patients at which liver biopsy was done [3]. Multiple studies agreed that clay colored stools had high sensitivity (86-94%) but low specificity ranging from as low as 39% to a high of 76% in predicting BA [15-17]. Our study also showed similar sensitivity (96%) and specificity (50%). Liu CS et al., found that serum Gamma glutamyl transferase concentration greater than 300 U/L was a useful diagnostic criteria for predicting BA patients with a diagnostic accuracy of 85% [18]. In our study also Gamma glutamyl transferase concentration was significantly elevated in BA compared to NH.
Small or absent gallbladder had a sensitivity range from 73% to 100% and specificity ranging from 67% to 100% in the detecting obstruction in BA [19]. In our study, collapsed or absent gallbladder was found in 76% of BA patients and 27% of NH patients. In a study by Yang JG et al., HIDA scan predicted BA with 88% sensitivity and 56% specificity [20]. Moyer V et al., found the sensitivity of this method for detecting BA to range from 83% to 100% while the specificity varied widely from 33 to 100% [19]. Findings of our study concurred with the above mentioned values. The initial false negative HIDA scan in NH can be attributed to various reasons like premature infants, very low birth weight newborn’s and children on total parenteral nutrition have less capacity to excrete the dye into the intestine [21]. In our study also eight patients had false negative in the HIDA scan. In these, four patients showed the excretion of dye on POC. POC is the gold standard for the diagnosis of BA [22]. In our study, based on the final diagnosis, POC was 100% sensitive and specific for discriminating BA from NH.
In their seminal study, Lee WS and Looi LM devised a pure histopathology based scoring system [3]. They concluded that the cutoff score of 7 or more had 88% sensitivity, 94% specificity, 91% positive predictive value and 92% negative predictive value for predicting BA [3]. There were five discrepant cases in their study. Two of them were falsely diagnosed as BA based on the score while the other three cases were falsely diagnosed as NH [3]. An Indian study also using the scoring system devised by Lee WS and Looi LM, found similar results [23]. Our study also found comparable results. There was a discrepancy between final diagnosis and the diagnosis based on scoring in three cases. One of them was falsely diagnosed as BA based on scores while the other two cases were falsely diagnosed as NH.
Lee WS and Looi LM found that among the histopathological features, moderate to marked bile ductular proliferation, portal expansion with bridging of more than 50% of the portal tracts and the bile plugs present in the bile ductules were identified in more than 50% cases of BA [3].
Among these, moderate to severe bile ductular proliferation had highest sensitivity and specificity in predicting BA. Periportal and diffuse hepatocytic swelling were present in more than 50% cases of intra hepatic cholestasis. While these had high sensitivity in predicting NH, giant cell transformation, neutrophilic infiltration, hepatocellular swelling, and lymphocytic infiltration had high specificity in predicting NH (97%, 91%, 88% and 79% respectively) [3]. Russo P et al., also drew similar conclusions in a research consortium setting [6]. We found similar results in our study with giant cell transformation and hepatocellular swelling having high sensitivity and specificity in predicting NH.
While assessing liver biopsies in our study, we observed infiltration by inflammatory cells both lymphocytes and neutrophils in majority of our patients. This may be related to the fact that in India, the principal aetiology responsible for NC is believed to be acquired infections [15]. Ultimately, we observed that lymphocytic infiltration was present in more than 50% of cases and had higher sensitivity and specificity in predicting BA than NH. This is contrary to the weightage in scoring given by Lee WS and Looi LM [3]. On the other hand, neutrophilic infiltration had nearly equal sensitivity and specificity and did not appear helpful in differentiating NH from BA. Thus, it is perhaps necessary that we lay less weightage to neutrophilic infiltration and more stress on lymphocytic infiltration in our setup and accordingly, modify the scoring system to make it more reliable.
We found that none of the histopathological features was, by themselves 100% sensitive and specific in diagnosing the aetiology of NC. Therefore, the use of scoring systems which assess multiple histological features is essential. In the present study among the various investigations helpful in the differentiation of BA, POC had 100% sensitivity and specificity. Stool colour and HIDA scan had high sensitivity and low specificity for predicting BA. USG had both the low sensitivity and specificity in predicting BA. Histopathological scoring had both high sensitivity and specificity for predicting BA, underscoring the importance of a detailed histomorphological evaluation of liver biopsy in all cases of NC.
Limitation
This study was done on 51 cases of NC, however, both HIDA scan and POC were not available in all the cases and in four cases of NH the final diagnosis was given based on the clinical, biochemical, imaging and histopathological features.
Conclusion
Histopathological scoring system (score of 7 or more) of liver biopsy was helpful to differentiate BA from NH and correlated with other clinical, biochemical and imaging features.