The Effect of Hydro-Alcoholic Extraction of Valerian on Number and Morphology of Raphe Magnus Nucleus in Astrocytes in Rat Model
Hatamijoni Sajad1, Rozbehi Amrollah2, Seyed Forootan Farzad3, Jafari Barmak Mehrzad4
1 Resident, Department of Orthopaedic, Ahvaz University of Medical Sciences, Ahvaz, Iran.
2 Associated professor, Cellular and Molecular Research Center, Yasuj University of Medical Sciences, Yasuj, Iran.
3 Ph.D of Biotechnology, Royan Institute of Biotechnology, Academic Center for Education Culture and Research, Isfahan, Iran.
4 Assistant Professor, Cellular and Molecular Research Center, Yasuj University of Medical Sciences, Yasuj, Iran.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Jafari Barmak Mehrzad, Assistant Professor, Cellular and Molecular Research Center, Yasuj University of Medical Sciences, Yasuj, Iran.
E-mail: mehrzadj14@gmail.com
Introduction
The valerian root extract has been used to treat sleep disorders, stress, depression, anxiety, muscle stiffness and tension. Astrocyte cells are involved in neural support, nutrition and protection. They regulate the transmission of electrical impulses within the brain.
Aim
The aim of the present study was to evaluate the effect(s) of valerian extract on the number and size of the astrocyte cells in raphe magnus nucleus of rats.
Materials and Methods
In the present experimental study, 40 male Wistar rats weighing 170-250 gm were randomly divided into four groups as follows: one control and three experimental groups. The control group received distilled water while animals in Group 2, 3 and 4 were gavaged by 300, 400 and 600 mg valerian root extract daily, respectively for two weeks. Astrocyte cells were stained with phosphotungstic acid. The size and number of astrocyte cells were calculated using the LS starter software. The collected data were analysed using SPSS statistical analysis with ANOVA and LSD tests.
Results
The mean number of astrocytes in experimental Groups 3 and 4 showed a significant increase in comparison with the control and experimental Group 2 (p-value <0.05). The mean diameter of astrocytes in all groups compared with the control group showed a significant decrease. Moreover, in Group 2, compared with Groups 3 and 4, the difference was statistically significant (p-value <0.05).
Conclusion
Aqueous extracts of valerian taken orally will increase the number of astrocytes in the raphe magnus.
In addition, administration of this extract reduced the diameter of astrocytes in nucleus raphe magnus, which is indicative of cell proliferation in the nucleus raphe magnus.
Animal model, Astrocyte cells, Valeriana root extact
Introduction
Reticular formation is a wide spread network in medulla oblongata and the brain stem. The raphe magnus nuclei are a moderate size cluster of nuclei found in the brain stem (interpeduncular anterior nucleus and medulla oblongata). Their axons are extended into rostral and caudal direction sporadically [1]. Raphe nuclei system is admitted as the most extensive and complex anatomical and neurochemical system in the mammalian brain [2,3]. The special feature of this nuclei is that the significant ratio of its neuron population is serotonergic; However, research shows that dopamine, neurotensin, substance P and enkephalins as neurotransmitters are released in the raphe nucleus. Neurons in the raphe nucleus are the main sources of the release of serotonin in the brain [4]. There are seven or eight raphe nuclei, that are concentrated in the center and around the brain stem. Axons of neurons in the raphe nuclei form a neurotransmitter system, reaching almost every part of the central nervous system. Axons of neurons in the raphe nuclei in the cerebellum and spinal cord ends lower, while higher nuclei axons in the brain are broadcasted. The nucleus raphe magnus releases serotonin when stimulated. Serotonergic action is terminated primarily via uptake of 5-hydroxytryptamine (5-HT) from the synapse [5-9]. The operation identified through a monoamine neurotransmitter called Serotonin Transporter (SERT) on parasympathetic neurons. Factors such as ecstasy {3,4-Methylenedioxymethamphetamine (MDMA)}, amphetamines, cocaine, dextromethorphan (a cough syrup), tricyclic antidepressants and selective serotonin reuptake inhibitors (SSRIs) prevent the reuptake of 5-HT. The 5-HT regulated activities such as sleep, appetite and libido. Hypothalamic suprachiasmatic nucleus serotonergic neurons projected along with norepinephrine and dopamine plays a role in regulating behavior. Acute stress increases serotonin temporarily, but chronic stress can drain reserves of serotonin [10,11].
Nervous tissue is composed of two types of cells: neurons responsible for receiving, transmitting and processing the message and release neurotransmitter and neuroglia responsible for nutrition, protection and supporting nerve cells, including astrocytes, oligodendrocytes, microglia and ependymal [12,13]. Astrocyte have important role in neurometabolism, maintaining consistency and rigidity of the nervous system, mediator uptake and prevention of electrical spreading and protection of brain and spinal cord from injury [14,15].
The valerian plant with the scientific name Valeriana officinalis is native to Europe, Asia and North America. The oily extract obtained from the dried root of this plant is useful in treating sleep disorder, stress, depression, anxiety disorder, muscle stiffness and seizure. Effective compounds in the plant including valepotriat, hydrovalepotriate and isovalepotriate which have a lot of benefits in pharmaceutical industry and are used as a sedative, anticonvulsant, hypnotic and also treatment of depression [16-18].
It has been reported that the hydroalcoholic extract of valerian root has no significant effect on number of neurons significantly [18].
Valeriana officinalis is used in the treatment of sleep disturbances, stress, depression, anxiety, muscle relaxant and also as an anticonvulsant, but no study has been conducted on the effect of valerian on the morphology of astrocytes, so the goal of present study was to evaluate the regulatory effects of hydroalcoholic extract of valerian root on the size and number of raphe magnus nuclei astrocytes in rat brain.
Materials and Methods
This experimental research was conducted under the approved conditions by the Institutional Animal Care and Use Committee (IACUC) and Ethics Committee of Yasuj University of Medical Science which conforms to the provisions of the Declaration of Helsinki (DOH, 2013). Fourty male Wistar rats with an average weight of 170-250 gm and age 2-2.5 months were collected randomly from medical science animal house. Animals were handled according to the guide for care and use of laboratory animals (Yasuj University of medical sciences) and maintained in a 12 hrs light: 12 hrs dark photoperiod under constant temperature (22±2°C) and relative humidity for two weeks. The rats were divided into four different subgroups as following:
Group 1 (control): were gavaged with distilled water during the study,
Group 2: were gavaged with 300 mg/kg,
Group 3: were gavaged with 400 mg/kg and
Group 4: were gavaged with 600 mg/kg of extract every day.
Valerian roots were prepared and dried under indirect sunlight and and then grinded. The 500 gm of the dried extract was soaked in water and ethanol (1:1 ratio) for 24 hours, then filtered two times, transferred to rotary machine for concentrating and separating additional solvent. Next, the extract was dried in an incubator, under vacuum pressure at 50°C. Afterwards, it was weighed and dissolved in distilled water two times to the final volume of 500 mL. Rats were gavaged by water or hydroalcoholic extract of valerian root daily for 14 days. Finally, the rats were anaesthetised using Ketamine injection and a solution of Heparin, normal saline, Paraformaldehyde (5%) and Glutaric acid (5%) were perfused through the animal’s heart. Rat brain was removed from the skull and transferred to plates containing formalin (10%). The brain tissue were processed by the tissue processing and paraffin embedded. ten micron sections were prepared from the blocks by microtome. The specimens were serially cut and one piece selected from each 10 pieces separately and stained with phosphotungistic acid. The Number and diameter of astrocytes were measured by a Olympus BX51 microscope and Olysia software.
Statistical Analysis
Data were analysed by SPSS (version 13.0). One way ANOVA test and LSD spatial test was used to compare the groups. Table data was presented based on the mean and standard deviation for each group. The differences in the proportions were considered signifcant when p-value <0.05.
Results
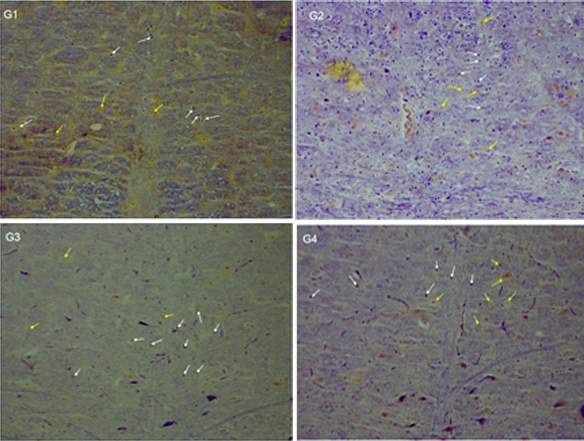
The mean number of astrocyte cells in the Group 3 and 4 which were gavaged by 400 and 600 mg/kg/day of valerian extract, compared with the Group 1 (control) and second Group 2 (gavaged by 300 mg/kg/day of valerian extract), indicated a statistically significant increase (p<0.05). The mean number of astrocyte cells in the Group 2 (gavaged by 300 mg/kg/day of valerian extract) did not indicate a significant difference compared to the Group 1 (control) (p>0.05) [Table/Fig-1]. The mean diameter of astrocytes in the Group 2,3,4 and in Group 1 (control) indicated a significant decrease. This reduced diameter was more in the Groups 3 and 4. Thereby, reducing the diameter of astrocytes in Group 4 compared with the Group 3 did not show a significant difference (p-value >0.05). The mean diameter of astrocytes in the Group 3 and 4 compared to the Group 2 indicated a significant decrease [Table/Fig-2,3]. Results show that astrocyte fibrils were blue and the neuronal cytoplasm turned yellow. Thus, increasing the number of astrocytes confirmed in Group 3 and 4 compared with the Group 1 (control). [Table/Fig-4].
Mean number of astrocyte cells in raphe magnus nuclei in different groups.
| Groups | Number of member | Mean number of astrocyte | p-value |
|---|
| Experimental Group-1 (Control) | 10 | 32.33±1.95 | 0.26 |
| Experimental Group-2 | 10 | 34.46±2.77 | 0.18 |
| Experimental Group-3 | 10 | 67.13±3.37* | 0.021 |
| Experimental Group-4 | 10 | 75.13±2.93* | 0.014 |
*Represent significant statistic different in compare with control group (p-value <0.05)
Mean diameter of astrocyte cells in raphe magnus nuclei in different groups.
| Groups | Number of member | Mean diameter of astrocyte | p-value |
|---|
| Experimental Group-1 (Control) | 10 | 10.25±0.63 | 0.17 |
| Experimental Group-2 | 10 | 6.45±0.37* | 0.042 |
| Experimental Group-3 | 10 | 4.53±0.9* | 0.021 |
| Experimental Group-4 | 10 | 3.9±0.34* | 0.013 |
*Represent significant statistic different in compare with control group (p-value <0.05)
Slide microscopic show neroun and astrocyte cells in raphe magnus nuclei in different groups (20X).
Neuron (yellow arrow), astrocyte (white arrow), phosphotungistic acid stain
Discussion
The mean number of astrocyte cells in the Groups 3 and 4 which were gavaged by 400 and 600 mg/kg/day of valerian extract, compared with the Group 1 (control) and Group 2 (gavaged by 300 mg/kg/day of valerian extract), indicated a statistically significant increase.
Much of the neurons of the raphe magnus nuclei are serotonergic. However, there were evidence of presence of neurotransmitters such as dopamine, neurotensin, substance P and enkephalin existence [5-9]. The anatomical differences between these cells in the various animals are very low, so that the data obtained from this study of rats can generalise to other animals and human [18,19].
The number of serotonergic cells in the raphe magnus nucleus was abundant [20,21]. Chemical or electrical stimulation of the raphe magnus nucleus caused the release of serotonin in the spinal cord. Valeriana officinalis are active ingredients in pharmaceutical industry and used as sedatives, anticonvulsants, hypnotics and also to treat depression and anxiety. Research shows that valerian affects the neurotransmitters and receptors [22,23].
In the present study, the mean number of astrocytes in the experimental Groups 3 and 4 compared to the Group1 (control) and the experimental group compared to the number of astrocytes in these two groups indicated no significant differences. In the present study, the mean number of astrocytes in the experimental Groups 3 and 4 compared to the Group 1 (control) and the experimental group 2 indicated significant differences. But the control group compared with the second group and the third experimental group compared with the fourth group indicated no statistical significant difference. Teaching and spatial learning can increase astrocyte cells in dental gyrus and this increase can be related to the duration of learning [24-26].
The researchers examined the effect of spatial teaching in the number of astrocytes in different area of the rat hippocampus and it has been reported that the spatial teaching may increase the number of astrocytes in different areas of the hippocampus [27-29]. The mean diameter of astrocytes in nucleus raphe magnus in the experimental group second, third and fourth in compared to the control group were not showing a statistically significant difference.
The mean diameter of astrocytes in nucleus raphe magnus in the third and fourth experimental groups compared to the second experimental group showed a significant decrease, but the third experimental group compared with the fourth group did not show a statistically significant difference. It can be concluded that valerian extract increases the proliferation of astrocytes in the raphe magnus [30-32].
Because the astrocytes population of this area were younger and therefore smaller, according to the findings, related to the number of astrocytes, the increase was justified. Astrocytes are positioned between the neurons and the capillaries and plays an important role in neuron energy metabolism [29-33].
Glycogen in the brain tissue is stored in large quantities in astrocytes. Astrocytes are neuroglia cells in the central neural tissue, which play an important role in the metabolism of materials, nutrition, waste removal and axonal neuronal conduction [28,29]. Recent studies have shown that astrocytes have active role in neuronal activity such as ion exchange, energy production, release of neurotransmitters and producing synapses [34]. Studies conducted so far have only supported the role of astrocytes to neurons in the central nervous system, but a recent study has reported a greater role for astrocytes in information processing [35]. This study indicated that astrocytes not only receive login information, but also transmit signals between neurons. The secretion of neurotransmitters such as serotonin interactions and GABA valerian is due to this fact [19-21].
Conclusion
Proliferation of astrocytes is to support the activity of neurons. So, this extract reduces the size of astrocytes in this area, all of which show the increased effect of this extract in astrocytes. Histochemistry for testing examination raphe magnus nucleus did not have sufficient financial means.
*Represent significant statistic different in compare with control group (p-value <0.05)
*Represent significant statistic different in compare with control group (p-value <0.05)
[1]. Di Ieva A, Grizzi F, Jelinek H, Pellionisz AJ, Losa GA, Fractals in the neurosciences, part I: general principles and basic neurosciencesNeuroscientist 2014 20(4):403-17.10.1177/107385841351392724362815 [Google Scholar] [CrossRef] [PubMed]
[2]. Lee HS, Kim MA, Valentino RJ, Waterhouse BD, Glutamatergic afferent projections to the dorsal raphe nucleus of the ratBrain Res 2003 963(1):57-71.10.1016/S0165-0173(02)00220-5 [Google Scholar] [CrossRef]
[3]. Hornung JP, The human raphe nuclei and the serotonergic systemChem Neuroanat 2003 26(4):331-43.10.1016/j.jchemneu.2003.10.002 [Google Scholar] [CrossRef]
[4]. Matthews GA, Nieh EH, Vander Weele CM, Halbert SA, Pradhan RV, Yosafat AS, Dorsal raphe dopamine neurons represent the experience of social isolationCell 2016 164(4):617-31.10.1016/j.cell.2015.12.04026871628 [Google Scholar] [CrossRef] [PubMed]
[5]. Rampon C, Jouvet M, Lower brainstem catecholamine afferents to the rat dorsal raphe nucleusJ Comp Neurol 1996 364(3):402-13.10.1002/(SICI)1096-9861(19960115)364:3<402::AID-CNE2>3.0.CO;2-8 [Google Scholar] [CrossRef]
[6]. Dorocic IP, Fürth D, Xuan Y, Johansson Y, Pozzi L, Silberberg G, A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nucleiNeuron 2014 83(3):663-78.10.1016/j.neuron.2014.07.00225102561 [Google Scholar] [CrossRef] [PubMed]
[7]. Monti JM, The structure of the dorsal raphe nucleus and its relevance to the regulation of sleep and wakefulnessSleep Med Rev 2010 14(5):307-17.10.1016/j.smrv.2009.11.00420153669 [Google Scholar] [CrossRef] [PubMed]
[8]. Trulson ME, Cannon MS, Raese JD, Identification of dopamine-containing cell bodies in the dorsal and median raphe nuclei of the rat brain using tyrosine hydroxylase immunochemistryBrain Res Bull 1985 15(2):229-34.10.1016/0361-9230(85)90142-X [Google Scholar] [CrossRef]
[9]. Allen GV, Cechetto DF, Serotoninergic and nonserotoninergic neurons in the medullary raphe system have axon collateral projections to autonomic and somatic cell groups in the medulla and spinal cordJ Comp Neurol 1994 350(3):357-66.10.1002/cne.9035003037533797 [Google Scholar] [CrossRef] [PubMed]
[10]. Gonulalan EM, Nemutlu E, Demirezer LO, Quality control of valerian products by HPLC fingerprint analysisPlanta Med 2016 82(S 01):S1-381.10.1055/s-0036-1597076 [Google Scholar] [CrossRef]
[11]. Colemana JA, Greena EM, Gouauxa E, X-ray structures and mechanism of the human serotonin transporterNature 2016 532(7599):334-39.10.1038/nature1762927049939 [Google Scholar] [CrossRef] [PubMed]
[12]. Leathwood PD, Chauffard F, Heck E, Munoz-Box R, Aqueous extract of valerian root (Valeriana officinalis L.) improves sleep quality in manPharmacol Biochem Behav 1982 17(1):65-71.10.1016/0091-3057(82)90264-7 [Google Scholar] [CrossRef]
[13]. Houghton PJ, The scientific basis for the reputed activity of valerianPharma Pharmacol 1999 51(5):505-12.10.1211/002235799177277210411208 [Google Scholar] [CrossRef] [PubMed]
[14]. Rezvani ME, Roohbakhsh A, Allahtavakoli M, Shamsizadeh A, Anticonvulsant effect of aqueous extract of valeriana officinalis in amygdala-kindled rats: possible involvement of adenosineJ Ethnopharmacol 2010 127(2):313-18.10.1016/j.jep.2009.11.00219900527 [Google Scholar] [CrossRef] [PubMed]
[15]. Yuan CS, Mehendale S, Xiao Y, Aung HH, Xie JT, Ang-Lee MK, The gamma-aminobutyric acidergic effects of Valerian and valerenic acid on rat brainstem neuronal activityAnesth Analg 2004 98(2):353-58.10.1213/01.ANE.0000096189.70405.A514742369 [Google Scholar] [CrossRef] [PubMed]
[16]. Jones S, Light AR, Serotoninergic medullary raphespinal projection to the lumbar spinal cord in the rat: a retrograde immunohistochemical studyJ Comp Neurol 1992 322(4):599-610.10.1002/cne.9032204131383285 [Google Scholar] [CrossRef] [PubMed]
[17]. Li Y, Zhong W, Wang D, Feng Q, Liu Z, Zhou J, Serotonin neurons in the dorsal raphe nucleus encode reward signalsNat Commun 2016 7:1050310.1038/ncomms1050326818705 [Google Scholar] [CrossRef] [PubMed]
[18]. Murphy K, Kubin ZJ, Shepherd JN, Ettinger RH, Valeriana officinalis root extracts have potent anxiolytic effects in laboratory ratsPhytomedicine 2010 17(8):674-78.10.1016/j.phymed.2009.10.02020042323 [Google Scholar] [CrossRef] [PubMed]
[19]. Sudati JH, Fachinetto R, Pereira RP, Boligon AA, Athayde ML, Soares FA, In vitro antioxidant activity of valeriana officinalis against different neurotoxic agentsNeurochem Res 2009 34(8):1372-79.10.1007/s11064-009-9917-819191025 [Google Scholar] [CrossRef] [PubMed]
[20]. Del Valle-Mojica LM, Ayala-Marín YM, Ortiz-Sanchez CM, Torres-Hernández BA, Abdalla-Mukhaimer S, Ortiz JG, Selective interactions of Valeriana officinalis extracts and valerenic acid with [3H] glutamate binding to rat synaptic membranesEvidence-Based Complementary and Alternative Medicine 2011 (2011):40359110.1155/2011/40359121584239 [Google Scholar] [CrossRef] [PubMed]
[21]. Ermine CM, Wright JL, Parish CL, Stanic D, Thompson LH, Combined immunohistochemical and retrograde tracing reveals little evidence of innervation of the rat dentate gyrus by midbrain dopamine neuronsFront Biol 2016 11(3):246-55.10.1007/s11515-016-1404-4 [Google Scholar] [CrossRef]
[22]. Müller LG, Salles L, Lins HA, Feijó PR, Cassel E, Vargas R, Effects of diene valepotriates from Valeriana glechomifolia on Na+/K+-ATPase activity in the cortex and hippocampus of micePlanta Med 2015 81(03):200-07.10.1055/s-0034-139620025615276 [Google Scholar] [CrossRef] [PubMed]
[23]. McDevitt RA, Tiran-Cappello A, Shen H, Balderas I, Britt JP, Marino RA, Serotonergic versus nonserotonergic dorsal raphe projection neurons: differential participation in reward circuitryCell Rep 2014 8(6):1857-69.10.1016/j.celrep.2014.08.03725242321 [Google Scholar] [CrossRef] [PubMed]
[24]. Torterolo P, Sampogna S, Chase MH, Hypocretinergic and non-hypocretinergic projections from the hypothalamus to the REM sleep executive area of the ponsBrain Res 2013 1491:68-77.10.1016/j.brainres.2012.10.05023122879 [Google Scholar] [CrossRef] [PubMed]
[25]. Curzi ML, The role of corticotropin-releasing hormone in REM sleep regulation: a possible mechanism through the cholinergic systemMünchen, Ludwig-Maximilians-Universität 2014 2014 [Google Scholar]
[26]. Semwal P, Kapoor T, Anthwal P, Sati B, Thapliyal A, Herbal extract as potential modulator and drug for synaptic plasticity and neurodegenerative disordersInt J Pham Sci Rev Res 2014 25(1):69-79. [Google Scholar]
[27]. Pilerood SA, Prakash J, Nutritional and medicinal properties of valerian (valeriana officinalis) herb: a reviewInt J Food, Nutr Diet 2013 1(1):25-32. [Google Scholar]
[28]. Santos G, Giraldez-Alvarez LD, Ávila-Rodriguez M, Capani F, Galembeck E, Neto AG, SUR1 receptor interaction with hesperidin and linarin predicts possible mechanisms of action of Valeriana officinalis in parkinsonFront Aging Neurosci 2016 8:9710.3389/fnagi.2016.0009727199743 [Google Scholar] [CrossRef] [PubMed]
[29]. Devi VS, Rao MG, Valeriana wallichii-a rich aroma root plantWorld J Pharm Pharm Sci 2014 3(9):1516-25. [Google Scholar]
[30]. Pinheiro ML, Alcântara CE, de Moraes M, de Andrade ED, Valeriana officinalis L. for conscious sedation of patients submitted to impacted lower third molar surgery: a randomized, double-blind, placebo-controlled split-mouth studyJ Pharm Bioallied Sci 2014 6(2):109-14.10.4103/0975-7406.12917624741279 [Google Scholar] [CrossRef] [PubMed]
[31]. Dyayiya N, Oyemitan I, Matewu R, Oyedeji O, Oluwafemi S, Nkeh-Chungag B, Chemical analysis and biological potential of valerian root as used by herbal practitioners in the eastern cape provinceAfrican J Trad Comple Alter Med 2015 13(1):114-22.10.4314/ajtcam.v13i1.16 [Google Scholar] [CrossRef]
[32]. Hornung JP, The neuronatomy of the serotonergic systemHandbook of Behavioral Neuroscience 2010 21:51-64.10.1016/S1569-7339(10)70071-0 [Google Scholar] [CrossRef]
[33]. Dietz BM, Mahady GB, Pauli GF, Farnsworth NR, Valerian extract and valerenic acid are partial agonists of the 5-HT5a receptor in vitroMol Brain Res 2005 138(2):191-97.10.1016/j.molbrainres.2005.04.00915921820 [Google Scholar] [CrossRef] [PubMed]
[34]. Falkowska A, Gutowska I, Goschorska M, Nowacki P, Chlubek D, Baranowska-Bosiacka I, Energy metabolism of the brain, including the cooperation between astrocytes and neurons, especially in the context of glycogen metabolismInt J Mol Sci 2015 16(11):25959-81.10.3390/ijms16112593926528968 [Google Scholar] [CrossRef] [PubMed]
[35]. Smith SJ, Do astrocytes process neural information?Progress in Brain Research 1992 94:119-36.10.1016/S0079-6123(08)61744-6 [Google Scholar] [CrossRef]