Introduction
Lymph Node Metastasis (LNM) is the most unfavourable outcome and a criterion of inoperability in Cholangiocarcinoma (CCA). Computerised Tomography (CT) scans are commonly used for preoperative evaluation of LNM. However, the accuracy of CT scan in LNM detection has not been clearly evaluated.
Aim
The aim of this study was to evaluate the accuracy of CT scan for preoperative LNM detection in CCA.
Materials and Methods
This diagnostic test study was based on an ongoing prospective cohort study (Cholangiocarcinoma Screening and Care Program, CASCAP) in nine tertiary care hospitals in the Northeastern region of Thailand. The subjects were patients with suspected CCA based on ultrasound examination. CT scan was done to evaluate the lesion and LNM. For operable subjects, the lymph nodes were dissected and sent for pathological diagnosis. The results were then compared with the radiographic results.
Results
The CT scan accurately detected the presence or absence of LNM in 78 of 127 CCA subjects (61.4%, 95% CI 52.4-69.9), resulting in a sensitivity of 50.8% (95% CI 37.9-63.6) and a specificity of 71.9% (95% CI 59.2-82.4). The positive and negative predictive values were 64.0% (95% CI: 49.2-77.1) and 59.7% (95% CI: 47.9-70.8), respectively. The positive likelihood ratio was 1.81 (95% CI: 1.14-2.86) and the negative likelihood ratio was 0.69 (95% CI: 0.51-0.92).
Conclusion
The CT scan has limited accuracy in the preoperative diagnosis of LNM. Therefore, surgeons should be aware of the number of false positives in determining inoperable patients.
Introduction
Lymph node metastasis is the most unfavourable prognostic factor for CCA [1]. CCA patients with LNM had zero percent 5-year survival [2-6] compared with 35-72% 5-year survival in non LNM patients [6,7]. The median survival time of patients with LNM was only 5-22.9 months compared with 28-70 months in patients with non LNM [8-12].
The site of LNM in patients with CCA is dependent on the location of the tumour, for instance, the intrahepatic type mainly spread to the nodes in the hepatoduodenal ligament, then to the common hepatic artery nodes, para-aortic nodes, or retropancreatic nodes. The left peripheral or hilar type can spread along the left gastric nodes through the lesser curvature [13]. Preoperative detection of LNM is necessary for an appropriate surgical procedure and treatment plan. Preoperative detection of LNM beyond the hepatoduodenal area, such as N2 lymph nodes, celiac, portocaval or para-aortic lymph nodes is also included as a criterion of inoperability in CCA [14, 15]. For instance, if LNM was detected the treatment plan would be changed to non operative methods or supportive treatment.
CT is a useful tool for preoperative evaluation. While, the quality of imaging has improved in recent years, the accuracy of the CT scans in preoperative evaluation is still problematic, especially for the assessment of the resectability of tumour and LNM [16,17]. Previous studies have been conducted to evaluate the sensitivity and specificity of CT scans in the detection of LNM in CCA, the estimation of sensitivity and specificity varied widely from 33.3 to 100% and 71.4 to 100%, respectively [15,18-21]. The variances could be due to small sample size with the previous largest study having only 55 cases [15]. It is therefore essential that the accuracy of CT scan in LNM detection in CCA patients be clearly evaluated. This study aims to assess the accuracy of CT scans in the preoperative evaluation of LNM in CCA using data from a large prospective cohort study in the Northeastern region of Thailand [22], which has the highest incidence of this disease worldwide [23].
Materials and Methods
This study was a diagnostic test study based on retrospective analysis of the data from an ongoing large cohort study carried out by the CASCAP [24]. CASCAP is a prospective cohort study comprising two cohorts. The first is the screening cohort: participants were Thais from the Northeastern region of the country who had at least one of the following risk factors were enrolled: 40 years or older, previous infection with liver fluke, past treatment with praziquantel, or known consumption of raw freshwater fish [25-27]. Ultrasonography was done by medical radiologists or trained general practitioners at least annually. The second cohort included symptomatic patients, such as abdominal pain, jaundice or abdominal mass, who were suspected of having CCA from ultrasonography. The later cohort represented a conventional cancer registry [22].
The CASCAP cohort is an ongoing cohort which was conducted in nine tertiary care hospitals. The main purpose of the CASCAP project is to control CCA. Therefore, this project is attempting to include as many participants as possible, so the sample size was not calculated. However, at the time of this study, 94,237 participants were included in the CASCAP cohort. The total number of participants in the CASCAP cohort and the details will be presented in the full report of the CASCAP.
The subjects with suspected CCA from both cohorts were examined by CT scans. Multiple detector row CT was used. All patients underwent triple-phase CT procedure, which consisted of precontrast, arterial dominant and portal dominant phases. In precontrast phase, 5 mm and 8 mm thick sections were acquired. Arterial dominant and portal venous dominant phases were examined after 120 mL of non ionic contrast material (Iopromide, Ultravist 370; Schering, Berlin, Germany) was administered at a rate of 4.0 mL/second. Helical phase CT scans were obtained. The scanning parameters for multidetector row CT involved a gantry rotation time of 0.5-0.8 second with a 4×1.25 mm or 8×1.25 mm detector configuration, 2.5 mm section thickness, pitch of 1.0-1.5, 3 mm reconstruction interval for phases, 150 mAs, 120 kVP, and a matrix of 512×512. For arterial phase scanning, a delay 13 seconds was used after the maximal attenuation of the aorta reached 100 HU with bolus tracking. After completion of arterial phase scanning, a delay of 30 seconds was used for portal venous phase scanning.
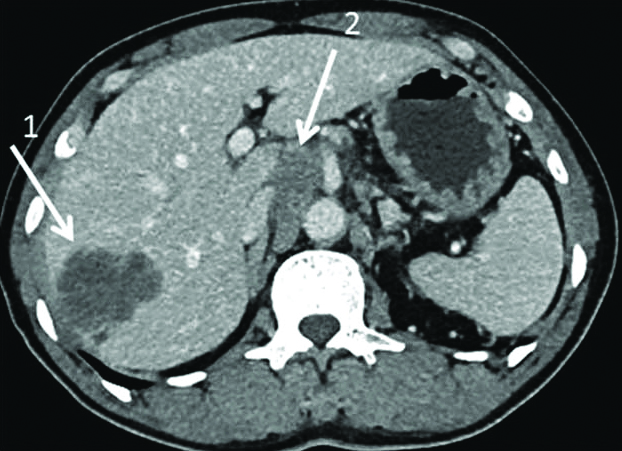
LNM was diagnosed by board-certified radiologists using morphological criteria, namely, nodal size greater than 10 mm in short axis diameter, central necrosis, and hyperattenuating enhancement following intravenous contrast medium injection [15] during the portovenous phase of scanning [Table/Fig-1].
Intrahepatic mass forming type cholangiocarcinoma in right lobe liver (arrow 1), enlargement of celiac and aortocaval nodes (arrow 2).
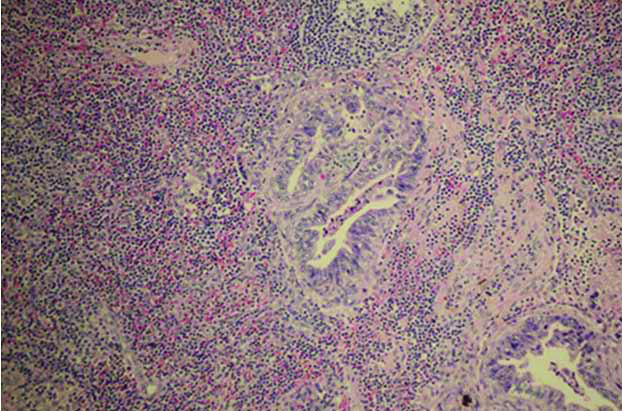
The treatment plans were designed by consensus between the surgeon and the subject after the counselling processes was completed. The risk and benefit of surgery were discussed including the prognosis after the surgical treatment. In the surgically treated patients, the operation types and plans were discussed with the surgeons before the procedure. The time gap between imaging and surgery was around one to two months. Lymph nodes dissections were done in the subjects with suspected LNM by the surgeons such as enlargement or abnormal consistency of lymph node. The dissected lymph nodes were subsequently sent for pathological examination. The pathological diagnosis of LNM was done by consensus of two board-certified pathologists [Table/Fig-2]. The CT scans and pathological results were compared by using the pathological result as the “gold standard” and CT scans result as the “diagnostic test”. We then calculated the diagnostic parameters.
Lymph node metastasis (Haematoxylin-eosin stain; 40X).
This study was conducted according to the principles of Good Clinical Practice (Chapter 2 of the International Conference of Harmonized Tripartite Guideline for Good Clinical Practice), the declaration of Helsinki, national laws and regulations about clinical studies. CASCAP was approved by Khon Kaen University Ethics Committee for Human Research.
Statistical Analysis
The patients’ characteristics and tumour types were presented as numbers and percentages. Diagnostic performances of the CT scans using pathological diagnosis results as the gold standard were calculated with their 95% Confidence Intervals (95% CI). The parameters included the accuracy, sensitivity, specificity, positive and negative predictive values and false positive and false negative rates. Such diagnostic performances were estimated for all and each type of CCA. The type of CCA was determined based on the American Joint Committee on Cancer (AJCC) criteria [28]. All statistical analyses were conducted using Stata 13 (StataCorp, College Station, TX).
Results
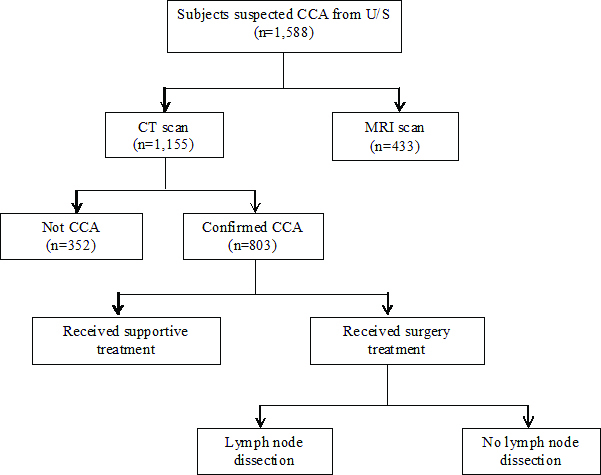
Of the 1,588 subjects who were suspected CCA from ultrasound examination, CT scan was done for 1,155 subjects and CCA was confirmed in 803 (69.5%). There were 561 (69.9%) subjects with intrahepatic CCA, 193 (24.0%) with perihilar CCA, and 49 (6.1%) with distal CCA.
In the CCA group confirmed by CT scans, 796 (99.1%) of the subjects had lymph node findings with 469 (58.9%) showing LNM. Of these, 195 subjects received surgical management and 127 subjects underwent lymph node dissection, with 63 (49.6%) subjects having LNM based on pathological diagnosis [Table/Fig-3].
Flow of subjects in the Cholangiocarcinoma Screening and Care Program (CASCAP).
CCA: Cholangiocarcinoma, U/S: Ultrasonography, CT: Computerised Tomography; MRI: Magnetic Resonance Imaging
In the 127 subjects undergoing lymph node dissection, the mean age was 58.8±8.31 years. A total of 84 subjects (66.1%) were male. Of these subjects, 82 subjects (64.6%) had intrahepatic, 33 subjects (26.0%) perihilar and 12 subjects (9.5%) intraductal CCA [Table/Fig-4].
Characteristics of patients and type of tumours presented as number and percentage unless specified otherwise.
| Characteristics | Subjects | (Percent) |
|---|
| Mean age±standard deviation | 58.8±8.31 years |
| Sex |
| Male | 84 | (66.1) |
| Female | 43 | (33.9) |
| Type of tumor |
| Intrahepatic | 82 | (64.6) |
| Perihilar | 33 | (26.0) |
| Intraductal | 12 | (9.5) |
The CT scans accurately detected the presence of LNM in 32 and the absence of LNM in 46 of the 127 CCA subjects (78/127, 61.4%, 95% CI 52.4-69.9) examined, resulting in a sensitivity of 50.8% (95% CI 37.9-63.6), and a specificity of 71.9% (95% CI 59.2-82.4). The positive and negative predictive values were 64.0% (95% CI 49.2-77.1) and 59.7% (95% CI 47.9-70.8), respectively. The positive likelihood ratio was 1.81 (95% CI 1.14-2.86) and the negative likelihood ratio was 0.69 (95% CI 0.51-0.92) [Table/Fig-5, 6]. The diagnostic performances of each subtypes of CCA are also presented in [Table/Fig-6].
Comparing the result of computerised tomography scan and pathological diagnosis (subjects).
| Result | Lymph node pathology | Total |
|---|
| Metastasis | Non metastasis |
|---|
| Computerisedtomography scan | Nodal metastasis | 32 | 18 | 50 |
| Non nodal metastasis | 31 | 46 | 77 |
| Total | 63 | 64 | 127 |
Diagnostic performance of computerised tomography scan in diagnosis of lymph node metastasis in different type of cholangiocarcinoma.
| Type of CCA | Sensitivity (95% CI) | Specificity (95% CI) | Positive predictiveValue (95% CI) | Negative predictivevalue (95% CI) | Positive Likelihood ratio (95% CI) | Negative likelihoodratio (95% CI) |
|---|
| Intrahepatic | 47.4 (31.0-64.2) | 75.0 (59.7-86.8) | 62.1 (42.3-79.3) | 62.3 (47.9-75.2) | 1.89 (1.03-3.49) | 0.70 (0.50-0.99) |
| Perihilar | 65.0 (40.8-84.6) | 53.8 (25.1-80.8) | 68.4 (43.4-87.4) | 50.00 (23.0-77.0) | 1.41 (0.72-2.75) | 0.65 (0.30-1.42) |
| Distal | 25.0 (0.63-80.6) | 85.7 (42.1-99.6) | 50.0 (1.26-98.7) | 66.7 (29.9-92.5) | 1.75 (0.15-21.0) | 0.88 (0.46-1.66) |
| Overall | 50.8 (37.9-63.6) | 71.9 (59.2-82.4) | 64.0 (49.2-77.1) | 59.7 (47.9-70.8) | 1.81 (1.14-2.86) | 0.69 (0.51-0.92) |
CCA: Cholangiocarcinoma, CI: Confidence Intervals.
Discussion
From this study’s data 1,155 subjects with suspected CCA had CT scanning performed, the sensitivity and specificity of CT scans, for the diagnosis of LNM in CCA, were 50.8% and 71.9%, respectively.
Only a few studies have reported the sensitivity and specificity of CT scans for LNM and there is no report from any Southeast Asian country. Six studies [15,18-21,29] compared the accuracy of CT scans for the diagnosis of LNM using pathological results as the “gold standard”; however, this was the largest study with a good analysis power to determine the accuracy of the CT scans. The reported sensitivity in our study is close to the reports from Lee HY et al., [15], Akamatsu N et al., [21] and Park TG et al., [29] which range from 50.0%-53.3%. These are lower than the results of Engels JT et al., who reported 100% sensitivity and 80% specificity [18], but higher than the sensitivity of 33.3%-35.7% found by Unno M et al., [19] and Watadani T et al., [20] [Table/Fig-6,7]. To increase the power of the analysis, we included subjects from these previous studies to increase the total to 286 subjects. The overall performance was 55.2% (95% CI 46.1-64.1) sensitivity, 78.3% (95% CI 71.1-84.4) specificity, 66.4% (95% CI 56.4-75.3) positive predictive values, and 69.2% (95% CI 62.0-75.9) negative predictive values. The positive and negative likelihood ratios were 2.54 (95% CI 1.82-3.54) and 0.57 (95% CI 0.46-0.71), respectively, with an accuracy of 68.2% (95% CI 62.4-73.5) [Table/Fig-7,8].
Literature review of diagnosis accuracy of computerised tomography scan in diagnosis of lymph node metastasis in cholangiocarcinoma.
| Source | Country | Number of patients | True Positive | False Positive | False Negative | True negative |
|---|
| Lee et al., [15] | South Korea | 55 | 8 | 2 | 7 | 38 |
| Engels et al., [18] | USA | 22 | 17 | 1 | 0 | 4 |
| Unno et al., [19] | Japan | 24 | 5 | 0 | 9 | 10 |
| Watadani et al., [20] | Japan | 13 | 2 | 2 | 4 | 5 |
| Akamatsu et al., [21] | Japan | 22 | 3 | 4 | 3 | 12 |
| Park et al., [29] | South Korea | 23 | 2 | 8 | 2 | 11 |
| Present study | Thailand | 127 | 32 | 18 | 31 | 46 |
| Total | | 286 | 69 | 35 | 56 | 126 |
Sensitivity, specificity, accuracy, predictive value and likelihood ratio of computerized tomography scan in diagnosis of lymph node metastasis in cholangiocarcinoma from literature review.
| Source | Sensitivity (95% CI) | Specificity (95% CI) | Positive predictive value (95% CI) | Negative predictive value (95% CI) | Positive likelihoodratio (95% CI) | Negative likelihoodratio (95% CI) |
|---|
| Lee et al., [15] | 53.3 (26.7-78.7) | 95.0 (83.1-99.2) | 80.0 (44.4-96.9) | 84.4 (70.5-93.5) | 10.7 (2.55-44.6) | 0.49 (0.28-0.85) |
| Engels et al.,[18] | 100 (80.3-100) | 80.0 (28.8-96.7) | 94.4 (72.6-99.1) | 100 (40.2-100) | 5.00 (0.87-28.9) | |
| Unno et al., [19] | 35.7 (12.9-64.8) | 100 (69.0-100) | 100 (48.0-100) | 52.6 (28.9-75.5) | | 52.6 (28.9-75.5) |
| Watadani et al., [20] | 33.3 (5.33-77.3) | 71.4 (29.3-95.5) | 50.0 (8.30-91.7) | 55.6 (21.4-86.0) | 1.17 (0.23-5.95) | 0.93 (0.45-1.95) |
| Akamatsu et al., [21] | 50.0 (12.4-87.6) | 75.0 (47.6-92.6) | 42.9 (10.4-81.3) | 80.0 (51.9-95.4) | 2.00 (0.62-6.42) | 0.67 (0.29-1.56) |
| Park et al., [29] | 50.0 (8.30-91.7) | 57.9 (33.5-79.7) | 20.0 (3.11-55.6) | 84.6 (54.5-97.6) | 1.19 (0.39-3.61) | 0.86 (0.30-2.47) |
| Present study | 50.8 (37.9-63.6) | 71.9 (59.2-82.4) | 64.0 (49.2-77.1) | 59.7 (47.9-70.8) | 1.81 (1.14-2.86) | 0.69 (0.51-0.92) |
| Overall | 55.2 (46.1-64.1) | 78.3 (71.1-84.4) | 66.4 (56.4-75.3) | 69.2 (62.0-75.9) | 2.54 (1.82-3.54) | 0.57 (0.46-0.71) |
Our concern is for the subjects who tested positive for LNM by CT scans, especially those with metastasis beyond the hepatoduodenal area which was included in the criteria for inoperable cases [14,15,30] to be managed only by supportive and non-surgical treatment. However, the data from all subjects from this and previous studies demonstrated that the false positive rate of CT scans in the diagnosis of LNM is 21.7%. Therefore, we recommended that surgeons consider the apparent detection of LNM by CT scans to be only one criterion for inoperability. These data are compatible with that from a recent study that found the patient’s survival rate with lymph node enlargement was improved after hepatectomy [31,32].
The cause of the limited sensitivity and accuracy of CT scans was the criterion for assessing lymph nodes based on the nodal size assessed in the axial short-axis. This assessment method has limited accuracy because of its inability to detect microscopic disease in normal size nodes and to distinguish benign enlarged lymph nodes from malignant lymph nodes [33]. This limitation has significant clinical implications for CCA treatment, especially for the selection of operable or inoperable subjects.
The authors recommend further studies on the benefit of using new modalities in radiographic techniques such as metabolic mapping of lymph nodes [34], and methods to improve the accuracy of CT scans.
Limitation
The results of this study should be interpreted with caution because of several limitations. First, lymph node pathology, which is the gold standard of this study, was determined in only some surgical subjects. Second, the location, numbers and size of lymph nodes from radiography and surgery were not determined due to retrospective analysis of data. Third, the CT scanning was carried out in many centers with different machines, techniques and radiologists, indicating the possibility of variation in data collection. Finally, the time gap of about one to two months between the CT scans and the operation might affect the disease progression.
Conclusion
The CT scan has limited sensitivity and accuracy for the LNM diagnosis in CCA. Therefore, surgeons should be aware of the number of false positives in determining which patients should be defined as inoperable.
CCA: Cholangiocarcinoma, CI: Confidence Intervals.
[1]. Adachi T, Eguchi S, Lymph node dissection for intrahepatic cholangiocarcinoma: a critical review of the literature to dateJ Hepatobiliary Pancreat Sci 2014 21(3):162-8.10.1002/jhbp.3024027075 [Google Scholar] [CrossRef] [PubMed]
[2]. Igami T, Ebata T, Yokoyama Y, Sugawara G, Takahashi Y, Nagino M, Staging of peripheral-type intrahepatic cholangiocarcinoma: appraisal of the new TNM classification and its modificationsWorld Journal of Surgery 2011 35(11):2501-09.10.1007/s00268-011-1242-021879422 [Google Scholar] [CrossRef] [PubMed]
[3]. Ercolani G, Vetrone G, Grazi GL, Aramaki O, Cescon M, Ravaioli M, Intrahepatic cholangiocarcinoma: primary liver resection and aggressive multimodal treatment of recurrence significantly prolong survivalAnnals of Surgery 2010 252(1):107-14.10.1097/SLA.0b013e3181e462e620531002 [Google Scholar] [CrossRef] [PubMed]
[4]. Nanashima A, Shibata K, Nakayama T, Tobinaga S, Araki M, Kunizaki M, Relationship between microvessel count and postoperative survival in patients with intrahepatic cholangiocarcinomaAnnals of Surgical Oncology 2009 16(8):2123-29.10.1245/s10434-009-0494-519434454 [Google Scholar] [CrossRef] [PubMed]
[5]. Yedibela S, Demir R, Zhang W, Meyer T, Hohenberger W, Schonleben F, Surgical treatment of mass-forming intrahepatic cholangiocarcinoma: an 11-year Western single-center experience in 107 patientsAnnals of Surgical Oncology 2009 16(2):404-12.10.1245/s10434-008-0227-119037702 [Google Scholar] [CrossRef] [PubMed]
[6]. Yamashita Y, Taketomi A, Morita K, Fukuhara T, Ueda S, Sanefuji K, The impact of surgical treatment and poor prognostic factors for patients with intrahepatic cholangiocarcinoma: retrospective analysis of 60 patientsAnticancer Research 2008 28(4C):2353-59. [Google Scholar]
[7]. Sulpice L, Rayar M, Boucher E, Pele F, Pracht M, Meunier B, Intrahepatic [7]cholangiocarcinoma: impact of genetic hemochromatosis on outcome and overall survival after surgical resectionJournal of Surgical Research 2013 180(1):56-61.10.1016/j.jss.2012.10.05123183056 [Google Scholar] [CrossRef] [PubMed]
[8]. Cho SY, Park SJ, Kim SH, Han SS, Kim YK, Lee KW, Survival analysis of intrahepatic cholangiocarcinoma after resectionAnnals of Surgical Oncology 2010 17(7):1823-30.10.1245/s10434-010-0938-y20165987 [Google Scholar] [CrossRef] [PubMed]
[9]. de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessmentJ Clin Oncol 2011 29(23):3140-45.10.1200/JCO.2011.35.651921730269 [Google Scholar] [CrossRef] [PubMed]
[10]. Ali SM, Clark CJ, Zaydfudim VM, Que FG, Nagorney DM, Role of major vascular resection in patients with intrahepatic cholangiocarcinomaAnnals of Surgical Oncology 2013 20(6):2023-28.10.1245/s10434-012-2808-223263702 [Google Scholar] [CrossRef] [PubMed]
[11]. Saxena A, Chua TC, Sarkar A, Chu F, Morris DL, Clinicopathologic and treatment- related factors influencing recurrence and survival after hepatic resection of intrahepatic cholangiocarcinoma: a 19-year experience from an established Australian hepatobiliary unitJ Gastrointest Surg 2010 14(7):1128-38.10.1007/s11605-010-1203-120467830 [Google Scholar] [CrossRef] [PubMed]
[12]. Chang ME, Lei HJ, Chen MH, Yeh YC, Li CP, Hung YP, Evaluation of prognostic factors and implication of lymph node dissection in intrahepatic cholangiocarcinoma: 10-year experience at a tertiary referral centerJ Chin Med Assoc 2017 80(3):140-46.10.1016/j.jcma.2016.09.01028169208 [Google Scholar] [CrossRef] [PubMed]
[13]. Tsuji T, Hiraoka T, Kanemitsu K, Takamori H, Tanabe D, Tashiro S, Lymphatic spreading pattern of intrahepatic cholangiocarcinomaSurgery 2001 129(4):401-07.10.1016/S0039-6060(01)49159-8 [Google Scholar] [CrossRef]
[14]. Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BJ, Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinomaAnnals of Surgery 2001 234(4):507-17.discussion 17-1910.1097/00000658-200110000-0001011573044 [Google Scholar] [CrossRef] [PubMed]
[15]. Lee HY, Kim SH, Lee JM, Kim SW, Jang JY, Han JK, Preoperative assessment of resectability of hepatic hilar cholangiocarcinoma: combined CT and cholangiography with revised criteriaRadiology 2006 239(1):113-21.10.1148/radiol.238305041916467211 [Google Scholar] [CrossRef] [PubMed]
[16]. Ruys AT, van Beem BE, Engelbrecht MR, Bipat S, Stoker J, Van Gulik TM, Radiological staging in patients with hilar cholangiocarcinoma: a systematic review and meta-analysisThe British Journal of Radiology 2012 85(1017):1255-62.10.1259/bjr/8840530522919007 [Google Scholar] [CrossRef] [PubMed]
[17]. Kloek JJ, van Delden OM, Erdogan D, ten Kate FJ, Rauws EA, Busch OR, Differentiation of malignant and benign proximal bile duct strictures: the diagnostic dilemmaWorld Journal of Gastroenterology 2008 14(32):5032-38.10.3748/wjg.14.503218763286 [Google Scholar] [CrossRef] [PubMed]
[18]. Engels JT, Balfe DM, Lee JK, Biliary carcinoma: CT evaluation of extrahepatic spreadRadiology 1989 172(1):35-40.10.1148/radiology.172.1.25449242544924 [Google Scholar] [CrossRef] [PubMed]
[19]. Unno M, Okumoto T, Katayose Y, Rikiyama T, Sato A, Motoi F, Preoperative assessment of hilar cholangiocarcinoma by multidetector row computed tomographyJ Hepatobiliary Pancreat Surg 2007 14(5):434-40.10.1007/s00534-006-1191-417909710 [Google Scholar] [CrossRef] [PubMed]
[20]. Watadani T, Akahane M, Yoshikawa T, Ohtomo K, Preoperative assessment of hilar cholangiocarcinoma using multidetector-row CT: correlation with histopathological findingsRadiation Medicine 2008 26(7):402-07.10.1007/s11604-008-0249-418769997 [Google Scholar] [CrossRef] [PubMed]
[21]. Akamatsu N, Sugawara Y, Osada H, Okada T, Itoyama S, Komagome M, Diagnostic accuracy of multidetector-row computed tomography for hilar cholangiocarcinomaJournal of Gastroenterology and Hepatology 2010 25(4):731-37.10.1111/j.1440-1746.2009.06113.x20074166 [Google Scholar] [CrossRef] [PubMed]
[22]. Khuntikeo N, Chamadol N, Yongvanit P, Loilome W, Namwat N, Sithithaworn P, Cohort profile: cholangiocarcinoma screening and care program (CASCAP)BMC Cancer 2015 15:45910.1186/s12885-015-1475-726054405 [Google Scholar] [CrossRef] [PubMed]
[23]. Green A, Uttaravichien T, Bhudhisawasdi V, Chartbanchachai W, Elkins DB, Marieng EO, Cholangiocarcinoma in north east Thailand: a hospital-based studyTropical and Geographical Medicine 1991 43(1-2):193-98. [Google Scholar]
[24]. Available from: http://www.cascap.in.th [Google Scholar]
[25]. Chang JS, Tsai CR, Chen LT, Medical risk factors associated with cholangiocarcinoma in Taiwan: a population-based case-control studyPLoS One 2013 8(7):e6998110.1371/journal.pone.006998123894567 [Google Scholar] [CrossRef] [PubMed]
[26]. Charbel H, Al-Kawas FH, Cholangiocarcinoma: epidemiology, risk factors, pathogenesis, and diagnosisCurrent Gastroenterology Reports 2011 13(2):182-87.10.1007/s11894-011-0178-821271364 [Google Scholar] [CrossRef] [PubMed]
[27]. Kamsa-ard S, Laopaiboon M, Luvira V, Bhudhisawasdi V, Association between praziquantel and cholangiocarcinoma in patients infected with Opisthorchis viverrini: a systematic review and meta-analysisAsian Pacific Journal of Cancer Prevention 2013 14(11):7011-16.10.7314/APJCP.2013.14.11.701124377641 [Google Scholar] [CrossRef] [PubMed]
[28]. Koerkamp BG, Wiggers JK, Allen PJ, Busch OR, D’Angelica MI, DeMatteo RP, American Joint Committee on Cancer staging for resected perihilar cholangiocarcinoma: a comparison of the 6th and 7th editionsHPB (Oxford) 2014 16(12):1074-82.10.1111/hpb.1232025267346 [Google Scholar] [CrossRef] [PubMed]
[29]. Park TG, Yu YD, Park BJ, Cheon GJ, Oh SY, Kim DS, Implication of lymph node metastasis detected on 18F-FDG PET/CT for surgical planning in patients with peripheral intrahepatic cholangiocarcinomaClinical Nuclear Medicine 2014 39(1):01-07.10.1097/RLU.0b013e3182867b9924335565 [Google Scholar] [CrossRef] [PubMed]
[30]. Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Guidelines for the diagnosis and management of intrahepatic cholangiocarcinomaJournal of Hepatology 2014 60(6):1268-89.10.1016/j.jhep.2014.01.02124681130 [Google Scholar] [CrossRef] [PubMed]
[31]. Adachi T, Eguchi S, Beppu T, Ueno S, Shiraishi M, Okuda K, Prognostic Impact of Preoperative Lymph Node Enlargement in Intrahepatic Cholangiocarcinoma: A Multi-Institutional Study by the Kyushu Study Group of Liver SurgeryAnnals of Surgical Oncology 2015 22(7):2269-78.10.1245/s10434-014-4239-825582737 [Google Scholar] [CrossRef] [PubMed]
[32]. Buettner S, van Vugt JLA, Gaspersz MP, Coelen RJS, Roos E, Labeur TA, Survival after resection of perihilar cholangiocarcinoma in patients with lymph node metastasesHPB (Oxford) 2017 19(8):735-40.10.1016/j.hpb.2017.04.01428549744 [Google Scholar] [CrossRef] [PubMed]
[33]. Padhani A, Lymph nodes. In: Nicholson T, editorRecommendations for cross- sectional imaging in cancer management 2014 second edLondonThe Royal College of Radiologists [Google Scholar]
[34]. Annunziata S, Caldarella C, Pizzuto DA, Galiandro F, Sadeghi R, Giovanella L, Diagnostic accuracy of fluorine-18-fluorodeoxyglucose positron emission tomography in the evaluation of the primary tumor in patients with cholangiocarcinoma: a meta-analysisBioMed Research International 2014 2014:24769310.1155/2014/24769324955351 [Google Scholar] [CrossRef] [PubMed]