Acute Disseminated Encephalomyelitis: A Rare Complication of Varicella Zoster Infection in an Adult
Jahnvi Dhar1, Lokesh Kumar2, Pranav Ish3, S Anuradha4
1 Postgraduate Resident, Department of Medicine, Maulana Azad Medical College, New Delhi, India.
2 Ex Resident, Department of Medicine, Maulana Azad Medical College, New Delhi, India.
3 Ex Resident, Department of Medicine, Maulana Azad Medical College, New Delhi, India.
4 Director Professor, Department of Medicine, Maulana Azad Medical College, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. S Anuradha, Director Professor, Department of Medicine, Maulana Azad Medical College, Bahadur Shah Zafar Marg, New Delhi-110002, India.
E-mail: drsanuradha@gmail.com
Acute Disseminated Encephalomyelitis (ADEM) is a mutifocal, demyelinating disease of the brain and spinal cord of acute onset. It usually occurs after viral infections or post vaccination. Its occurrence in immunocompetent middle-aged adults, that too without cerebral dysfunction following Varicella Zoster Virus (VZV) infection is extremely rare. Here, we report a case of a 42-year-old female who developed ADEM after an episode of VZV infection. The patient responded dramatically to corticosteroids and made complete neurological recovery. Though she had extensive lesions on her brain MRI, she had no symptoms/signs related to brain involvement clinically. To the best of our knowledge, such a presentation has not been reported in literature so far.
Chicken pox, Encephalitis, Neurological complications
Case Report
A 42-year-old female with known β-thalassaemia trait, presented with complaints of sudden onset weakness of bilateral lower limbs and inability to pass urine for one day. Ten days prior to this complaint, she had high grade fever with erythematous, macular and vesicular rashes on the trunk, face and upper limb. She did not report any joint pains, ophthalmological complaints or gangrene. On examination, she had pallor and generalised skin lesions showing crusting and scab formation, characteristic of healing varicella zoster (chicken pox) lesions. There was no prior history of VZV infection and she was not immunised for VZV.
Neurological examination revealed bilateral upper motor neuron type paraplegia with sensory level at D7 (seventh dorsal spinal cord segment) level. The tone and reflexes in upper limbs were normal. The plantar response was extensor bilaterally. There was initial history of retention of urine with normal bowel functions. There were no meningeal or cerebellar signs. The patient did not experience seizures, altered behaviour and was conscious and oriented to time, place and person with normal cerebral functions (MMSE 27/30). The respiratory, cardiovascular and abdominal examinations were normal.
Investigations showed haemoglobin of 7.8gm/dL, MCV-50.3 fL, normal total leucocyte count (5600/cu mm) and platelet counts (3.45 lacs/cumm). The Erythrocyte Sedimentation Rate (ESR) was 46 mm in 1st hour. Peripheral blood smear showed red cells with anisopoikilocytosis and presence of microcytic hypochromic cells, elliptical cells and tear drop cells indicative of the previously diagnosed thalassemia trait. Blood sugar, renal and liver function tests were normal. Cerebrospinal Fluid (CSF) examination revealed lymphocytic pleocytosis with a total 10 cells, protein 85 mg/dL and sugar 91 mg/dL. VZV antibody (IgM) in the CSF was positive. Testing for tubercular bacilli in CSF by acid fast staining and Polymerase Chain Reaction (TB-PCR) was negative. There were no oligoclonal bands. Tests for Antinuclear Antibody (ANA), anti-Ro antibody, anti-La antibody and aquaporin-4 antibody (for NMO) were negative. Serological testing for Human Immunodeficiency Virus (HIV), Hepatitis B virus (HBsAg), Hepatitis C Virus (anti HCV IgG) and dengue virus (IgG, IgM and NS1 antigen) was negative. The X-ray of the chest, X-rays of the dorso-lumbar spine and ultrasonography of the abdomen and pelvis was normal. Fundoscopy was also normal.
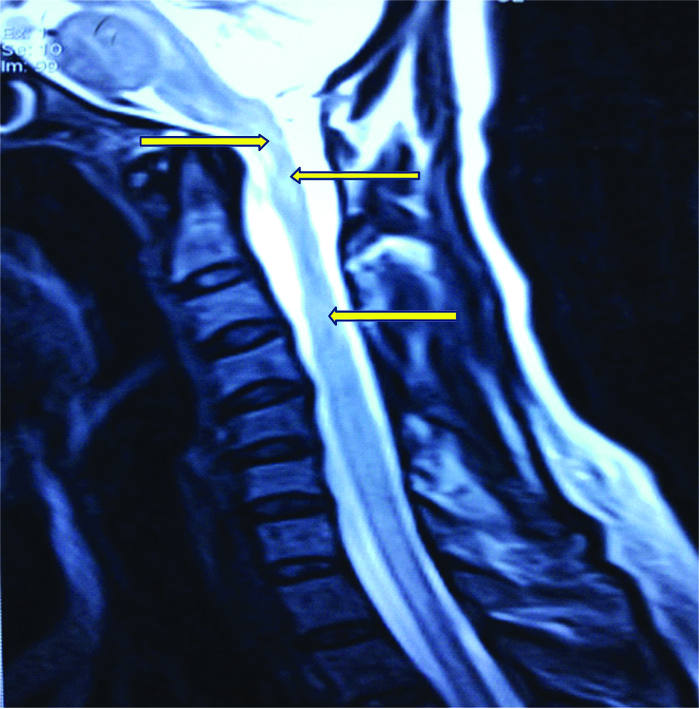
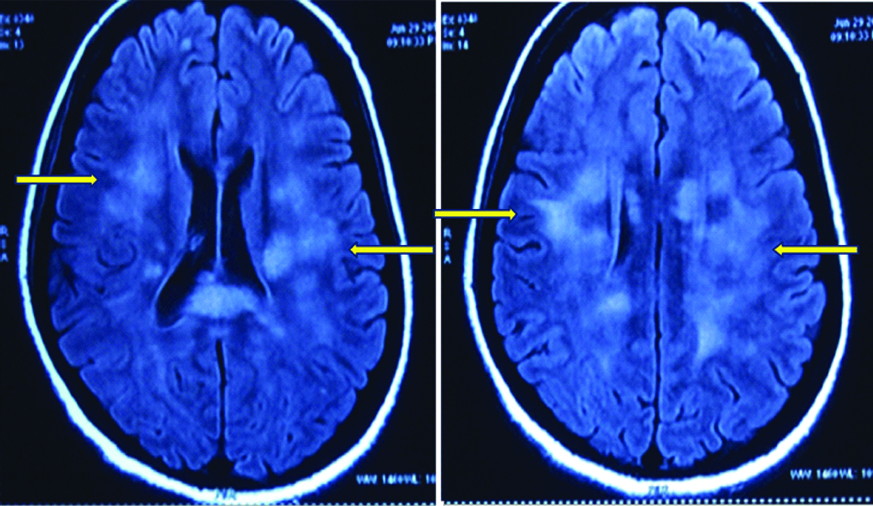
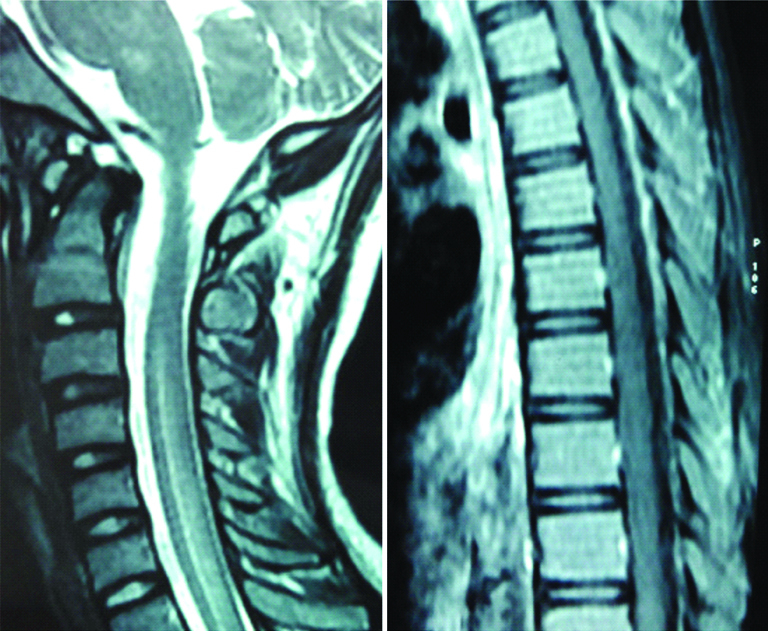
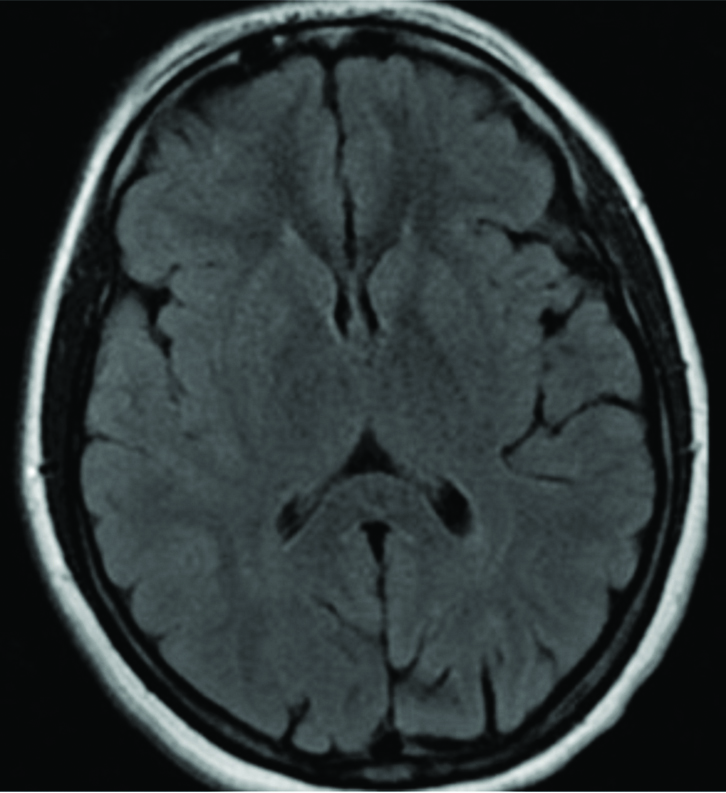
Magnetic Resonance Imaging (MRI) of the brain and spinal cord was undertaken and it revealed bilaterally symmetrical, hyperintense signal changes on T2-weighted and Fluid Attenuated Inversion Recovery (FLAIR) sequences in the pons, periventricular region, corpus callosum, bilateral frontal, subcortical and ganglio-thalamic regions. MRI of the spinal cord showed diffuse ill defined hyperintense signals in the cervical and the dorsal column on T2-weighted image without any cord swelling or contrast enhancement [Table/Fig-1a,b]. Based on the combination of clinical features and radiological findings in the setting of an antecedent VZV infection, a final diagnosis of ADEM (post VZV infection) was made. She was given injection methylprednisolone 1 gm daily for five days along with injection, Acyclovir 500 mg q8h. The patient showed remarkable recovery and her motor functions, sensory symptoms and bladder dysfunction improved within 10 days. Repeat MRI brain and spine after three months showed complete resolution of findings as compared to the previous MRI [Table/Fig-2a,b].
MRI Spine of the patient at the time of diagnosis demonstrating T2W hyperintensities noted in the cervical and dorsal cord.
MRI brain at the time of diagnosis showing multiple focal white matter hyperintensities on T2 FLAIR.
MRI spine (T2W images) after three months of presentation showing no obvious abnormality with complete resolution of the hyperintensities in the cervical and dorsal cord (in comparison with the first MRI spine).
MRI Brain (normal): complete resolution of the white matter hyperintensities after 3 months of treatment (in comparison with the initial film).
Discussion
ADEM is an immune mediated demyelinating disorder of CNS, that usually occurs within six days to four weeks after viral infections. The course of illness is monophasic and generally has an excellent response to corticosteroids [1]. It is most commonly seen in children and young adults, where the prognosis is favourable. Very few cases have been reported in literature in adult patients (an estimated incidence of 0.01-0.03%) and the clinical course, response to treatment and outcome of these patients is unclear [2].
Its annual incidence is around 0.4-0.8 per 100,000 population [3]. VZV is a neurotropic human herpes virus. It has well recognised CNS complications (2 per 10,000 cases) which include encephalitis and cerebellar ataxia but rare complications like Acute Transverse Myelitis (ATM), aseptic meningitis, Guillain-Barre Syndrome (GBS), meningoencephalitis, ventriculitis, optic neuritis and stroke have also been described [4]. VZV is a rare cause of ADEM with a reported incidence of 0.5-1 in 100,000 VZV affected population [3]. These figures have been mainly reported in the paediatric population. The prevalence of ADEM is usually higher in the younger population because of the increased frequency of viral infections and vaccination in this population. The proposed mechanisms for post VZV ADEM include either molecular mimicry or direct inflammatory damage to myelinated neurons by the VZV [5].
The diagnosis of post varicella ADEM is usually made with a combination of clinical features and radiological findings, but this can be supplemented with detection of VZV in the CSF and/or blood with the help of serological diagnosis and PCR. VZV serology by ELISA has been shown to have a sensitivity of 99.3% and a specificity of 100% [6]. White matter lesions on the MRI is seen in a number of diseases: infective (e.g., HIV, syphilis, viral encephalitis), inflammatory (e.g., ADEM, MS, neurosarcoidosis), metabolic (Wernicke’s encephalopathy and Vitamin B12 deficiency), vasculitis, mitochondrial diseases. Thus white matter lesions on the MRI are suggestive of ADEM though not specific for the condition.
Post VZV ADEM rarely presents in the middle-aged to elderly population and due to the paucity of cases reported in the literature, the prognosis in this age group is unknown. Only isolated case reports are available on the presentation of ADEM in immunocompetent adults with lack of guidelines on their management. The patient usually presents after 1-3 weeks of the illness with encephalopathy and focal neurological deficits with a downhill course [3]. A prospective study of 40 patients of ADEM was conducted by Schwarz S et al., [7] to determine the possibility of identifying patients who eventually develop clinical and radiological MS. In these patients, oligoclonal bands were found in 65% of patients and on follow up, 14 patients had developed clinically definite MS. Around 25% of patients of ADEM are known to develop Multiple Sclerosis (MS) within five years of the first presentation of ADEM but in majority of the individuals, the neurological dysfunction does not progress beyond three months [5]. As ADEM and MS are both probably immunologically mediated, infectious agents like varicella may trigger the episode of ADEM that may progress to MS later [8]. Corticosteroids are the cornerstone of management in these cases, with a timely diagnosis resulting in a full recovery. In a case report by Wang PN et al., [9] three cases of ADEM were described in elderly age group who showed fair to good recovery after corticosteroid administration. The patients who develop recurrences or fail to respond to the first line treatment were managed with IVIG (immunoglobulins) and plasma exchange [5].
In the present case, ADEM occurred in a middle age female due to underlying VZV infection. β-thalassaemia trait was an incidental association. At the time of peak neurological deficit, the patient was conscious, oriented, bed ridden and paraparetic with an indwelling urinary catheter. Although her MRI brain showed extensive white matter lesions, there were no clinical signs and symptoms suggestive of brain involvement, which makes it a very unusual presentation. Furthermore, in this patient, subsequent MRIs confirmed no evidence of new contrast enhancing lesions, with follow up MRI six months later showing complete resolution of a monophasic demyelinating event. With timely institution of high dose corticosteroids, the patient made a complete neurological recovery.
Post varicella ADEM in adult with a clinical presentation of isolated spinal cord involvement but radiological evidence of extensive brain involvement was a very unusual feature of our case.
Conclusion
ADEM is a rare disease of the CNS that is mainly a diagnosis of exclusion and post varicella ADEM is usually a disease of childhood. It relies heavily on neuroimaging which may be normal at the onset. It is important to differentiate ADEM from MS as early institution of therapy may alter the latter’s course. In all cases of ADEM, a thorough search should be made for any preceding illness or history of vaccination.
[1]. Menge T, Kieseier BC, Nessler S, Hemmer B, Hartung HP, Stüve O, Acute disseminated encephalomyelitis: an acute hit against the brainCurr Opin Neurol 2007 20(3):247-54.10.1097/WCO.0b013e3280f31b4517495616 [Google Scholar] [CrossRef] [PubMed]
[2]. Garg RK, Acute disseminated encephalomyelitisPostgraduate Medical Journal 2003 79(927):11-17.10.1136/pmj.79.927.1112566545 [Google Scholar] [CrossRef] [PubMed]
[3]. Noorbakhsh F, Johnson RT, Emery D, Power C, Acute disseminated encephalomyelitis: clinical and pathogenesis featuresNeurologic Clinics 2008 26(3):759-80.10.1016/j.ncl.2008.03.00918657725 [Google Scholar] [CrossRef] [PubMed]
[4]. Girija AS, Rafeeque M, Abdurehman KP, Neurological complications of chickenpoxAnn Indian Acad Neurol 2007 10(4):240-46.10.4103/0972-2327.37816 [Google Scholar] [CrossRef]
[5]. Lee YJ, Acute disseminated encephalomyelitis in children: differential diagnosis from multiple sclerosis on the basis of clinical courseKorean J Pediatr 2011 54(6):234-40.10.3345/kjp.2011.54.6.23421949517 [Google Scholar] [CrossRef] [PubMed]
[6]. Enzygnost Anti VZV/IgG. Enzyme immunoassay for the qualitative detection and quantitative determination of IgG antibodies to Varicella-Zoster virus (VZV) in human serum and plasma. Dade Behring, 2003, July edition, p8, Marburg, GmbH [Google Scholar]
[7]. Schwarz S, Mohr A, Knauth M, Wildemann B, Storch-Hagenlocher B, Acute disseminated encephalomyelitis: a follow-up study of 40 adult patientsNeurology 2001 56(10):1313-18.10.1212/WNL.56.10.131311376180 [Google Scholar] [CrossRef] [PubMed]
[8]. Smyk DS, Alexander AK, Walker M, Walker M, Acute disseminated encephalomyelitis progressing to multiple sclerosis: are infectious triggers involved?Immunol Res 2014 60(1):16-22.10.1007/s12026-014-8499-y24668297 [Google Scholar] [CrossRef] [PubMed]
[9]. Wang PN, Fuh JL, Liu HC, Wang SJ, Acute disseminated encephalomyelitis in middle-aged or elderly patientsEuro Neurol 1996 36(4):219-23.10.1159/0001172538814425 [Google Scholar] [CrossRef] [PubMed]