Drug induced thrombocytopenia is uncommon cause of life-threatening bleeding. The mechanisms may include bone marrow suppression or antibody-mediated platelet destruction.
We report the case of a two-year-old boy with incedental ingestion of Buparvaquone (BPQ), which is a veterinary drug against theileriosis in cattle that efficiently inhibits tachyzoite replication of parasite. He presented with severe Gastrointestinal (GI) bleeding, petechiae and purpura. Laboratory findings showed severe thrombocytopenia. Intravenous immunoglobulin (IVIg) and methylprednisolone were started for him. GI bleeding and thrombocytopenia was recovered. Therefore, in cases of life-threatening bleeding due to thrombocytopenia, we should also consider poisioning from drugs.
Case Report
A previously healthy two-year-old boy was referred to emergency room with upper and lower GI bleeding, petechiae, and purpura throughout the body. The patient accidentally drank a cattle drug named BPQ about eight hours prior to admission. He had not history of taking any other drugs or vaccine within three weeks prior to this clinical onset. He also had not history of taking quinine containing beverages tonic water and bitter lemon, food, such as tahini (pulped sesame seeds) or herbal remedies.
The child was pale, with respiratory distress and diffuse petechiae and purpura was observed throughout his body. Lymphadenopathy and hepatosplenomegaly were not observed. Vital signs revealed that he had blood pressure 65 mmHg (systolic), respiratory rate 45 breaths/minute, temperature 36.7°C (orally), pulse 150 beats/minute, and O2 saturation 88% by pulse oximetry.
The haemoglobin and platelet count on admission were 5.5 gm/dL and 6×103/μL respectively. Laboratory studies are summarised in [Table/Fig-1].
Laboratory findings in our case report.
| Haematology | Result | Normal Reference | Chemistry | Result | Normal Reference |
|---|
| WBC | 6.2×103/μL | 5×103-12×103 | FBS | 88 mg/dL | 60-110 |
| Neutrophils | 45% | 20%-40% | BUN | 9 mg/dL | 5-17 |
| Lymphocytes | 53% | 60%-70% | Cr | 0.8mg/dL | 0.1-0.8 |
| Monocytes | 2% | 5%-8% | Na | 139mEq/L | 135-145 |
| RBC | 3.1×106/μL | 4.1×106-5.1×106 | K | 4.5mEq/L | 3.5-5.5 |
| Haemoglobin | 5.5 g/dL | 11-14 | Ca | 10.1 mg/dL | 8-12 |
| Haematocrit | 16.1% | 33-42 | Po4 | 5.2 mg/dL | 3.1-6 |
| MCV | 73.2 fL | 74-89 | PH | 7.35 | 7.35-7.45 |
| MCH | 25.5 pg | 27-33 | Hco3 | 22 mg/dL | 22-24 |
| MCHC | 32.5 g/dL | 32-36 | Pco2 | 37 torr | 35-40 |
| Platelet | 6×103/μL | 150×103-450×103 | Total Protein | 6.3 g/dL | 3.5-5 |
| Fibrinogen | 210 mg/L | 150-350 | Albumin | 4.6 g/dL | <41 |
| ESR | 20 mm/hour | <20 | ALT | 13 U/L | 3-60 |
| CRP | Negative | | AST | 26 U/L | 150-650 |
| MPV | 12 fL | 9-10 | ALP | 492 U/L | 0.1-1.1 |
| PT | 12 second | 11-12 | Total Bilirubin | 0.5 mg/dL | 0.01-0.3 |
| PTT | 32 second | 26-35 | Direct Bilirubin | 0.1 mg/dL | 200-450 |
| D-dimer | < 0.5 mg/L | < 0.5 | LDH | 510 U/L | |
WBC: White Blood Cell, RBC: Red Blood Cell, MCV: Mean Corpuscular Volume, MCH: Mean Corpuscular Hemoglobin, MCHC: Mean Corpuscular Hemoglobin Concentration, ESR: Erythrocyte Sedimentation Rate, CRP: C- Reactive Protein, FBS: Fasting Blood Sugar, ALT: Alanine Aminotransferase, AST: Aspartate Aminotransferase, ALP: Alkaline Phosphatase, LDH: Lactic Acid Dehydrogenase, PT: Prothrombin Time, PTT: Partial Thrombin Time, MPV: Mean Platelet Volume.
Due to respiratory distress and hypotension, he received oxygen 4 litre/minutes by mask, hydrated with normal saline and also, transfusion of pack cell (10 mL/kg) and platelet (2 bags every 12 hours) were done. Since the GI bleeding could not be controlled by platelet transfusion after 24 hours, IVIg (1 gm/kg intravenous infusion for two days), and methylprednisolone pulse (30 mg/kg intravenous infusion for three days) were started. The bleeding was decreased after 24 hours of starting medications. In addition, there was no decrease in haemoglobin levels after 24 hours of starting medication.
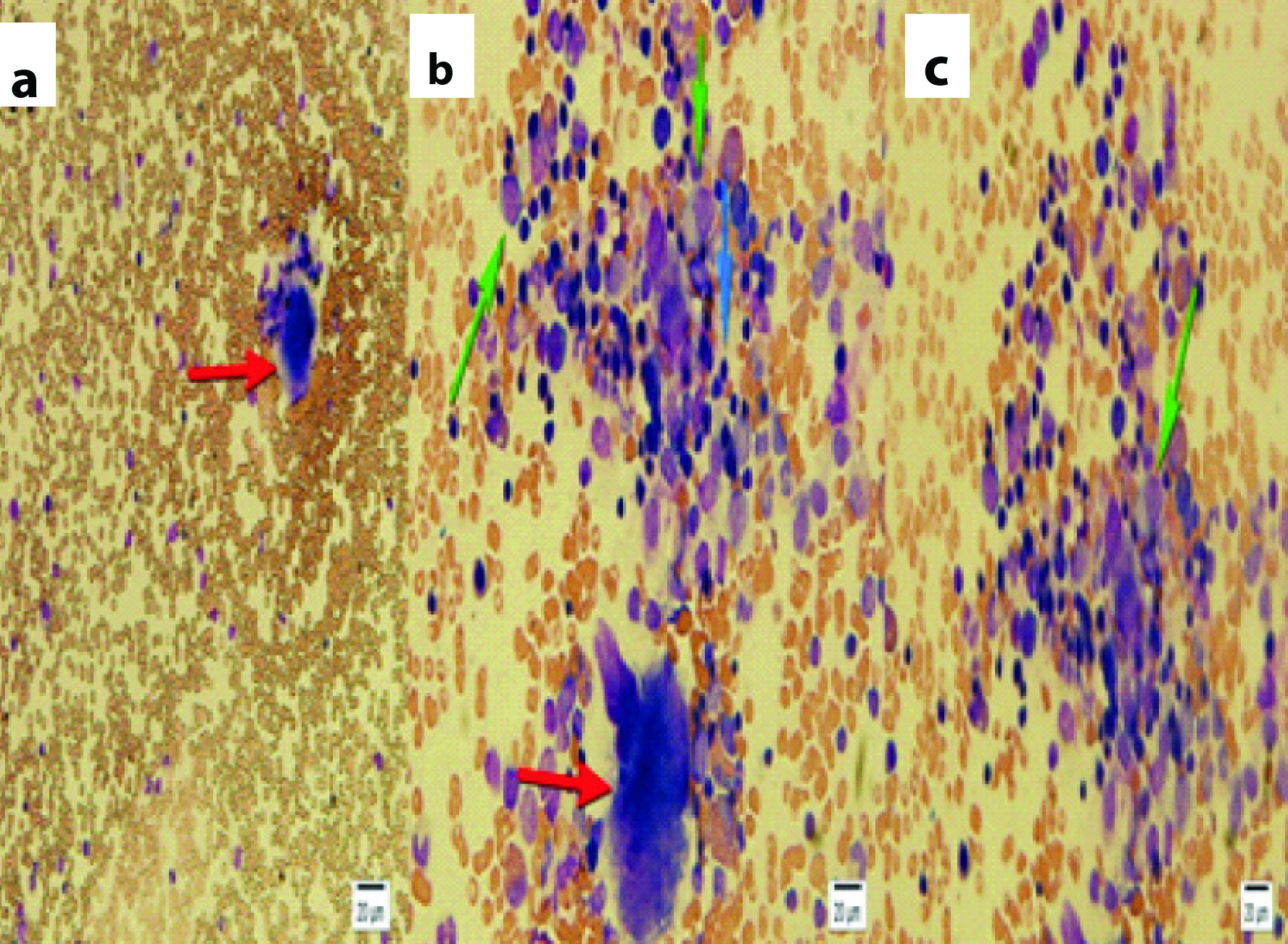
Bone marrow aspiration was performed to rule out bone marrow suppression or necrosis. Histopathology report showed adequate megakaryocyte count and mild hyper cellularity in erythroid precursor [Table/Fig-2]. GI endoscopy was performed which did not reveal any ulcerations or inflammation.
Bone marrow aspiration slides (Hematoxylin and Eosin stain) with polymorphic and mild hyper cellularity, megakaryocyte is present. Red arrows show megakaryocyte, blue arrows shows mature lymphocytes and green arrows show myelocyte (a-40X, b-100X, c-100X).
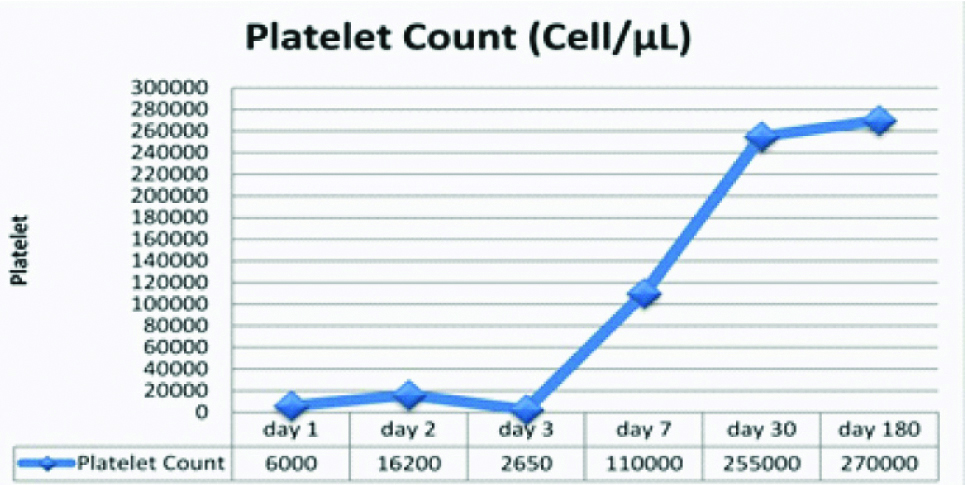
Eventually, the bleeding stopped after 48 hours of medication. Concisely, the sign and symptom related to BPQ poisoning in the studied patient included confusion and lethargy, hypotension, respiratory distress, tachycardia, and bleeding due to thrombocytopenia. In our studied patient, the platelet count got to normal level after one week. The patient was discharged after a week with good clinical condition. The patient completely recovered during the six months of follow up [Table/Fig-3]. A written informed consent was taken from infant’s legal guardian to publish and report the case.
Platelet count series in this case.
Discussion
Many drugs such as prescription drugs or herbal medicines, foods, beverages or substance use may lead to thrombocytopenia. Thrombocytopenia, which is developed by medicines, is called as Drug-Induced Immune Thrombocytopenia (DITP) that is a form of secondary Immune Thrombocytopenia (ITP). Some of the medicines with non immune mechanism may suppress bone marrow and give rise to thrombocytopenia, such as valacyclovir and chemotherapy drugs [1,2]. Another mechanism of DITP is conformational change in protein level, which leads to neo epitope exposure that stimulates antibody formation against platelet. The platelet antigens that are targeted by drug dependent antibodies consist of GpIIb/IIIa, GpIb-V-IX, GpV, platelet endothelial cell adhesion molecule-1 [3,4]. The list of prescribed drugs that can cause DITP and their ranking according to reported cases and level of evidence are shown in [Table/Fig-4,5] [5,6].
Methods for determining the level of Evidence in children with drug induced thrombocytopenia.
| Criteria for level of evidence |
|---|
| 1. Administration of suspected drug causes thrombocytopenia and complete recovery by cessation of drug |
| 2. The suspected drug was the only drug used before the beginning of thrombocytopenia or other drugs were continued after cessation of therapy with the suspected drug |
| 3. Excluded other causes of thrombocytopenia |
| 4. Re-exposure to the candidate drug lead to repeated thrombocytopenia |
| Levels of evidence for relationship between suspected drugs and thrombocytopenia in children |
| Definite | Level 1 | 4 criteria encountered |
| Probable | Level 2 | Criteria 1–3 encountered |
| Possible | Level 3 | Criterion 1 encountered |
| Unlikely | Level 4 | Criterion 1 not met |
List of prescribed drugs that may cause thrombocytopenia in children according to number of reported cases with definite or probable evidence.
| Drugs | Levels of evidence (Number of reports) |
|---|
| Definite evidence (Level 1) | Probable evidence (Level 2) |
|---|
| Carbamazepine | 3 | 6 |
| Amino salicylic acid | 1 | 0 |
| Dactinomycin | 1 | 0 |
| Ceftriaxone | 0 | 2 |
| Acetaminophen | 1 | 0 |
| Isotretinoin | 1 | 0 |
| Hepatitis B vaccine | 0 | 5 |
| Lupinus termis bean | 1 | 0 |
| Quinine | 1 | 2 |
| Lamivudine | 1 | 0 |
| Sodium stibogluconate | 1 | 0 |
| Trimethoprim/sulfamethoxazole | 0 | 6 |
| Phenytoin | 0 | 4 |
| Sulfasalazine | 1 | 0 |
Drugs with any reports of definite evidence (Level 1) or ≥ two reports with probable evidence (Level 2)
Although, DITP is a rare, all patients referred with new-onset thrombocytopenia, should be suspected for DITP [7]. In our case report, the patient incidentally consumed veterinary medicine that induced thrombocytopenia; however, it has been reported neither in animals nor in humans.
BPQ, described in the 1980s, has been widely used for veterinary practice against theileriosis in cattle. The cows with theileriosis were diagnosed based on clinical signs including jaundice, reduced milk, lethargy, and anemia. BPQ efficiently inhibited tachyzoite replication of parasite in theileriosis [8,9].
DITP is less common in children than in adults [5]. The onset of DITP symptoms varies from a few minutes to several days after using the drug. The thrombocytopenia is often severe with platelet count less than 20×103/μL in DITP [1,7,10]. There was less than 24 hours between the exposure of BPQ and thrombocytopenia in our case report.
The clinical signs of DITP are various from mild petechiae to severe bleeding, and it should be kept in mind that the risk of severe bleeding is higher in DITP [7]. In a study by McMahon CM (2014), the bleeding severity in DITP was 22% in 309 patients, and it was accompanied with risk of death even in the hospitalised patients [11].
In the present case, as there were no schistocytes or fragmented RBC in the peripheral blood, so drug induced thrombocytopenic microangiopathy was ruled out. In addition, we ruled out sepsis-induced thrombocytopenia due to the absence of leukocytosis, fever, and negative c-reactive protein. We did not consider disseminated intravascular coagulation, because the prothrombin time, partial thromboplastin time, fibrinogen, and D-Dimer level were normal.
In the DITP, unlike primary ITP, thrombocytopenia would resolve generally after drug discontinuation if there was no associated liver or renal problem [2]. Platelet count correction is important to confirm the diagnosis. The platelet count increment occurs after 1-2 days and returns to normal range in one week [1,10,12]. According to the bone marrow aspiration result in our case, it seems that the mechanism of BPQ induced thrombocytopenia was not bone marrow suppression; as the patient’s bone marrow had enough megakaryocytes [Table/Fig-5] and on the other hand high mean platelet volume in the peripheral blood smear.
Platelet transfusion should be considered as life threatening haemorrhage [1,13]. In severe bleeding diathesis that do not respond to platelet transfusion, IVIg should also be considered, as we recommended two doses of 1 gm/kg/day IVIg in two first days of hospitalisation [14]. Therefore, if there was severe bleeding in patients with DITP, treatment with methylprednisolone and IVIg might be helpful.
Conclusion
The clinicians should keep in mind that BPQ could be a probable cause of DITP in a case of thrombocytopenia. BPQ poisoning in humans is usually presented with thrombocytopenia and life threatening bleeding, which can be controlled by platelet transfusion, IVIg, methylprednisolone. In addition, supportive cares like blood transfusion.
WBC: White Blood Cell, RBC: Red Blood Cell, MCV: Mean Corpuscular Volume, MCH: Mean Corpuscular Hemoglobin, MCHC: Mean Corpuscular Hemoglobin Concentration, ESR: Erythrocyte Sedimentation Rate, CRP: C- Reactive Protein, FBS: Fasting Blood Sugar, ALT: Alanine Aminotransferase, AST: Aspartate Aminotransferase, ALP: Alkaline Phosphatase, LDH: Lactic Acid Dehydrogenase, PT: Prothrombin Time, PTT: Partial Thrombin Time, MPV: Mean Platelet Volume.
Drugs with any reports of definite evidence (Level 1) or ≥ two reports with probable evidence (Level 2)
[1]. Pedersen-Bjergaard U, Andersen M, Hansen PB, Drug-induced thrombocytopenia: clinical data on 309 cases and the effect of corticosteroid therapyEur J Clin Pharmacol 1997 52(3):183-89.10.1007/s0022800502729218924 [Google Scholar] [CrossRef] [PubMed]
[2]. Neylon AJ, Saunders PW, Howard MR, Proctor SJ, Taylor PR, Group NRH, Clinically significant newly presenting autoimmune thrombocytopenic purpura in adults: a prospective study of a population-based cohort of 245 patientsBr J Haematol 2003 122(6):966-74.10.1046/j.1365-2141.2003.04547.x12956768 [Google Scholar] [CrossRef] [PubMed]
[3]. Kroll H, Sun QH, Santoso S, Platelet endothelial cell adhesion molecule-1 (PECAM-1) is a target glycoprotein in drug-induced thrombocytopeniaBlood 2000 96(4):1409-14. [Google Scholar]
[4]. Aster RH, Curtis BR, McFarland JG, Bougie DW, Drug-induced immune thrombocytopenia: pathogenesis, diagnosis, and managementJ Thromb Haemost 2009 7(6):911-18.10.1111/j.1538-7836.2009.03360.x19344362 [Google Scholar] [CrossRef] [PubMed]
[5]. Reese JA, Nguyen LP, Buchanan GR, Curtis BR, Terrell DR, Vesely SK, Drug-induced thrombocytopenia in childrenPediatr Blood Cancer 2013 60(12):1975-81.10.1002/pbc.2468224038783 [Google Scholar] [CrossRef] [PubMed]
[6]. George JN, Aster RH, Drug-induced thrombocytopenia: pathogenesis, evaluation, and managementHematology Am Soc Hematol Educ Program 2009 :153-58.10.1182/asheducation-2009.1.15320008194 [Google Scholar] [CrossRef] [PubMed]
[7]. Warkentin TE, Drug-induced immune-mediated thrombocytopenia--from purpura to thrombosisN Engl J Med 2007 356(9):891-93.10.1056/NEJMp06830917329695 [Google Scholar] [CrossRef] [PubMed]
[8]. Müller J, Aguado-Martinez A, Manser V, Balmer V, Winzer P, Ritler D, Buparvaquone is active against Neospora caninum in vitro and in experimentally infected miceInt J Parasitol Drugs Drug Resist 2015 5(1):16-25.10.1016/j.ijpddr.2015.02.00125941626 [Google Scholar] [CrossRef] [PubMed]
[9]. Dhar S, Malhotra DV, Bhushan C, Gautam OP, Chemoimmunoprophylaxis with buparvaquone against theileriosis in calvesVet Rec 1987 120(15):37510.1136/vr.120.15.375-c3590595 [Google Scholar] [CrossRef] [PubMed]
[10]. Rousan TA, Aldoss IT, Cowley BD, Curtis BR, Bougie DW, Aster RH, Recurrent acute thrombocytopenia in the hospitalized patient: sepsis, DIC, HIT, or antibiotic-induced thrombocytopeniaAm J Hematol 2010 85(1):71-74.10.1002/ajh.2153619802882 [Google Scholar] [CrossRef] [PubMed]
[11]. McMahon CM, Cuker A, Hospital-acquired thrombocytopeniaHosp Pract (1995) 2014 42(4):142-52.10.3810/hp.2014.10.115125502138 [Google Scholar] [CrossRef] [PubMed]
[12]. George JN, Raskob GE, Shah SR, Rizvi MA, Hamilton SA, Osborne S, Drug-induced thrombocytopenia: a systematic review of published case reportsAnn Intern Med 1998 129(11):886-90.10.7326/0003-4819-129-11_Part_1-199812010-000099867731 [Google Scholar] [CrossRef] [PubMed]
[13]. Ray JB, Brereton WF, Nullet FR, Intravenous immune globulin for the treatment of presumed quinidine-induced thrombocytopeniaDICP 1990 24(7-8):693-95.10.1177/1060028090024007061695793 [Google Scholar] [CrossRef] [PubMed]
[14]. Benesch M, Kerbl R, Lackner H, Berghold A, Schwinger W, Triebl-Roth K, Low-dose versus high-dose immunoglobulin for primary treatment of acute immune thrombocytopenic purpura in children: results of a prospective, randomized single-center trialJ Pediatr Hematol Oncol 2003 25(10):797-800.10.1097/00043426-200310000-0001114528103 [Google Scholar] [CrossRef] [PubMed]