The DC is the second most common odontogenic cyst, accounting to approximately 20.3% of all epithelium-lined cysts of the jaws in Indian population [1]. It characteristically encloses a crown of an unerupted tooth and is attached to the neck of tooth at the Cementoenamel Junction (CEJ). The pathogenesis of this cyst is unknown, but has been postulated to develop from accumulation of fluid between the tooth crown and Reduced Enamel Epithelium (REE) [2]. It predominantly occurs in the second and third decades, with a slight male predilection [3]. DC is most prevalent in the third molar region and is therefore a common cause of a radiolucency associated with the crown of an impacted mandibular third molar [4]. Radiographically, the DC presents, usually, as a well-defined, unilocular, or occasionally multilocular radiolucency with corticated margins associated with the crown of an unerupted tooth. Some DC may result in considerable displacement of the involved tooth.
Microscopically, the DC presents with a thin flattened non-keratinised stratified squamous epithelial lining without rete ridge formation resembling REE [4]. DC are usually asymptomatic but may become symptomatic due to the large size or secondary infection. Occasionally, DC lining may show neoplastic transformation into benign odontogenic tumour or even a carcinoma [5,6]. A rapid increase in the size of the lesion should therefore be viewed with a high degree of suspicion for a possible aggressive benign or malignant neoplasm. Clinicopathologic studies on DC are sparse in the literature [3,7,8]. Nevertheless, there are many articles on odontogenic cyst in which DC were seen to a certain extent [9-12]. The present study aimed to determine the relative frequency of DC at SDM College of Dental Sciences and Hospital, India, over a period of 20 years (1989 to 2009), its clinicopathologic features and comment on the incidence of neoplastic transformation along with comparison of these data with previous reports.
Materials and Methods
The material for this study was obtained by retrospective review of the biopsy files of Department of Oral and Maxillofacial Pathology, SDM College of Dental Sciences and Hospital, Dharwad, Karnataka, India from January 1989 to December 2009, a span of 20 years. In the present study, inclusion of OKC as odontogenic cyst was based on WHO updated classification on 2017 where a major revision was done regarding OKC and Keratocystic Odontogenic Tumour (KCOT) from the previous classification. i.e., move KCOT back into the cyst category as OKC [13]. A total of 68 histologically diagnosed cases of DC were subjected to detailed analysis with regards to age, sex, site of involvement, clinical features, radiographic presentation, histopathological features and management. Haematoxylin and Eosin (H&E) stained sections and relevant special stained slides were reviewed by the authors to find any unusual features and to reconfirm the diagnosis/or modify where necessary.
Results
During the period, a total of 6400 biopsy specimens were received in Oral Pathology Department SDM college of Dental sciences, Dharwad, Karnataka, India. Of these, 68 cases (1.06% of all specimens) were diagnosed as DC among a total of 685 odontogenic cysts [Table/Fig-1]. The age of present presentation varied between 06-70 years with 25 cases occurring in children with an age range of 06-14 years. The highest prevalence of DC was found in patients aged 11-20 years (38.23%), followed by age range 21-30 years (25%) and 31-40 years (16.17%). Less than 7% of cases occurred above the age of 40 years. The average age of patients with DC was 22.76 years. 48 (70.58%) cases presented in males and the remaining 20 (29.41%) in females. Age distribution of DC for males and females are shown in [Table/Fig-2].
Distribution of odontogenic cysts seen over 20 year period.
| Type of Odontogenic Cyst | No. of Cases | % |
|---|
| Radicular cyst | 320 | 46.71 |
| Infected cyst/capsule | 201 | 29.34 |
| Odontogenic keratocyst | 80 | 11.67 |
| Dentigerous cyst | 68 | 9.93 |
| Residual cyst | 7 | 1.02 |
| Calcifying Odontogenic cyst | 5 | 0.72 |
| Glandular Odotogenic cyst | 2 | 0.29 |
| Lateral Periodontal cyst | 2 | 0.29 |
| Total | 685 | |
Age and sex distribution of dentigerous cyst.
| Age group (year) | Male | Female | Total (%) |
|---|
| 1-10 | 8 | 1 | 9 (13.23%) |
| 11-20 | 18 | 8 | 26 (38.23%) |
| 21-30 | 10 | 7 | 17 (25%) |
| 31-40 | 8 | 3 | 11 (16.17%) |
| 41-50 | 2 | 1 | 3 (4.41%) |
| 51-60 | 0 | 0 | 0 |
| 61-70 | 2 | 0 | 2 (2.94%) |
| Total | 48 | 20 | 68 |
Dentigerous cyst was predominantly seen in maxilla (40 cases 58.82%) compared to mandible (28 cases 41.17%) [Table/Fig-3,4].
Distribution of dentigerous cyst.
| Site | No. of cases (%) |
|---|
| Maxillary | Anterior region | 35 |
| Posterior region | 5 |
| Total | 40 (58.82) |
| Mandibular | Anterior region | 7 |
| Posterior region | 21 |
| Total | 28 (41.17) |
| Total | 68 |
Age distribution of dentigerous cyst related to sex and location.
| Male | Female | Mandible | Maxilla | Total |
|---|
| No. of cases | 48 | 20 | 28 | 40 | 68 |
| Mean age (year) | 22.64 | 23.05 | 26.32 | 20.27 | 22.76 |
| Age range (year) | 6-70 | 7-42 | 7-70 | 8-45 | 6-70 |
| % of cases | 70.58% | 29.41% | 41.17% | 58.82% | |
Maxillary lesions were common in the anterior region (35/68, 51.47%) and predominantly seen associated with canines (25/68). Only five cases were found involving maxillary posterior region [Table/Fig-3]. DC in mandible were common in posterior region (21/68, 30.88%), most of which were associated with third molars (15/68, 22.05%). Mandibular lateral incisor (1/68, 1.47%), mandibular canine (6/68, 8.82%) and premolars (6/68, 8.82%) were the other teeth involved by DC. Supernumerary teeth (SN) were the third most common teeth affected by DC (10/68, 14.70%) and all the associated SN teeth (mesiodens 6/10, 60%) were in maxillary anterior region.
Maxillary lesions occurred at a younger age (mean 20.27 years) than mandibular DC (mean age 26.32 years) Most of the cases presented as an asymptomatic swelling (43/68, 63.23%) and were accidentally discovered on radiographs.
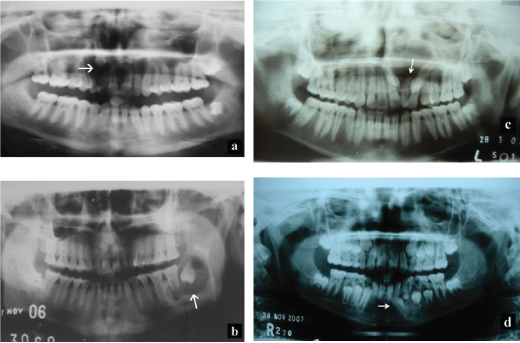
Radiographic examination revealed a unilocular radiolucency with well-defined border surrounding the crown of an unerupted tooth in majority of the cases [Table/Fig-5a-d]. A single case showed irregular outline. Well circumscribed radiolucency surrounding the crown of unerupted succedaneous tooth with partially formed root were also observed in few cases (7/68, 10.3%); five cases involving first premolar and two cases involving second premolar, with the radiolucency extending to involve the root apices of overlying deciduous molars. Few cases showed unilocular radiolucency along with foci of radiopacities (9/68, 13.2%). Involvement of coronoid process and maxillary sinus was evident in two separate cases. Buccal cortical plate expansion (9) lingual cortical plate expansion (5), displacement of the associated tooth either to lower border of mandible or to the maxillary sinus and resorption of associated teeth (5) were the other noted findings. Based on clinical and radiographic findings, 45 (66.17%) cases were provisionally diagnosed as DC with others being diagnosed as either radicular cyst 7 (10.29%), unicystic ameloblastoma 11 (16.17%) or as AOT 5 (7.35%). Some of the cases presented with coexistence of OKC, radicular cyst and odontome in other locations of the jaws.
a) Radiographs depicting unilocular radiolucency with scalloped border with an impacted impacted maxillary canine, b) mandibular third molar, c) supernumerary tooth and d) mandibular premolar.
All the lesions were surgically removed along with associated unerupted teeth and sent for histopathological evaluation. Macroscopic examination of the excised specimen in most of the cases revealed predominantly well encapsulated soft tissue masses associated with a tooth. The masses were greyish to white in colour, soft in consistency and with smooth border. On sectioning, the mass was seen to be attached to the neck CEJ of the tooth. Some cases exhibited proliferations in the inner wall of these cysts giving a suspicion of neoplastic transformation (11/68, 16.17%). Few cases showed root resorption of the associated tooth (5/68).
Histopathological examination revealed a thin non-keratinised stratified squamous to cuboidal epithelial lining of two to four cell layer thickness resembling reduced enamel epithelium in most cases [Table/Fig-6a,b].
Photomicrographs demonstrating histopathologic features of dentigerous cyst. Thin non-keratinised epithelial lining of dentigerous cyst and fibrous connective tissue capsule; a) thin epithelial lining, areas of hyalinisation and chronic inflammatory cells; b) epithelial lining exhibiting squamous metaplasia; c) Mucous metaplasia; d) Adenomatoid odontogenic tumour arising from dentigerous cyst lining; e) Ameloblastoma arising from DC; f) (H&E, original magnification 100X).
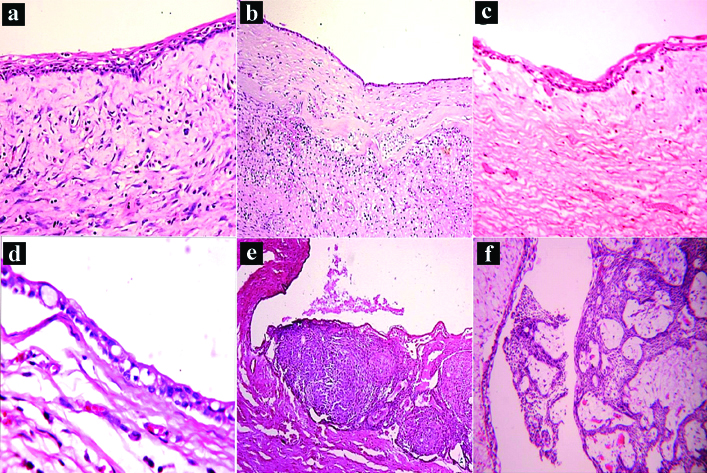
Proliferation of the epithelial lining was evident in three cases. In few of the maxillary, cases (3) there was also evidence of pseudostratified ciliated columnar epithelial lining which was not in any way related to the maxillary sinus. There was evidence of REE showing metaplastic change to squamous epithelium in three cases [Table/Fig-6c] and mucous metaplasia in a single case [Table/Fig-6d].
Cystic capsule was fibrous to fibrocellular and showed presence of chronic inflammatory cells mainly lymphocytes, cholesterol clefts and multinucleated giant cells in secondarily infected DC. Other features were presence of calcification, odontogenic rests and areas of hyalinisation.
Few odontogenic cysts showed non-keratinised stratified squamous epithelial lining proliferating in arcading pattern with intense chronic inflammatory cell infiltration in the capsule. Clinically, these were seen associated with deciduous and their succedaneous teeth.
Some of the DC (16/68, 24%) showed epithelial lining with neoplastic transformation into either AOT (9/68, 13.2%) [Table/Fig-6e] or ameloblastoma (7/68, 10.2%) [Table/Fig-6f]. All the cases were surgically excised under local anaesthesia and followed up subsequently for a period of six months to three years with no recurrences being reported.
Discussion
The relative frequency of DC vary from 17.1% to 35.5% among all jaw cysts [2,14]. In the present study, DC accounted for 8.25% of all jaw cyst, which is much lower than majority of studies in literature. Previous studies have reported that DC to be the second most prevalent among odontogenic cysts [9-16]. However, the present study showed DC to be the third most common odontogenic cyst as reported by Tay AB after radicular cyst and OKC [17].
The presenting age range in our study was 6-70 years, which is in close agreement with other studies ranging from 4-75 years [8,18]. However, Zang LL et al., Lin HP et al., and Jone AV et al., reported an age range between 10-29 years, 6-99 years and 17-89 years respectively [3,7,14]. These findings suggest that the DC can occur over a wide age range. The mean age of 22.76 years for DC in this study is lower when compared to other studies. Yeo JF et al., Jones AV et al., Mourshed F reported 30.2, 32.3 and 41 years respectively [8,14,18]. Also, keeping with DC elsewhere, the peak frequency of occurrence in our patients was in the second decade of life followed by third decade [3,8,19]. This is because the mandibular third molars and the maxillary permanent canines are the most commonly impacted teeth, and are most frequently associated with DC. Development of these cysts occur at an early stage, at the time of eruption (Maxillary canine 11-12 years (second decade) and mandibular molars 17 to 21 years (second and third decade). That is attributed to the fact that the erupting tooth exert pressure on the follicle leading to cyst formation [4]. Its prevalence in fifth decade was reported by Jones AV et al., which differs from findings of other studies in the literature [14]. Generally DC are slightly more frequent in males than in females. Our study was in agreement with the literature, but showed a much higher incidence (70.52%) in males compared to others which ranged from 48% to 65.09% [3,9,10,14-16]. However, Prockt AP et al., reported a female predominance in his study [11]. However, the reason for the gender predilection is not clear. Daley TD et al., suggested that it may be related to a smaller jaw size and a greater tendency for prophylactic extraction of third molars in females [2].
In relation to localisation, a preference for maxilla (58.82%) especially anterior region, followed by mandibular (41.17%) posterior region was observed. These figures are in agreement with very few authors but contrast to the findings of others in the literature [8,12,20], who reported mandible being the most frequently affected site [3,4,7,9,11,18].
Although, DC can involve any unerupted tooth, they usually involve third molars/canines and rarely involve premolars, deciduous teeth, supernumerary teeth, or odontomes. The present study showed predominance of DC in relation to maxillary canine (24/68, 35.29%) followed by mandibular third molars. (15/68, 22.05%).
Its association with mandibular lateral incisor is a very rare finding. Involvement of SN teeth in the present study was also much higher compared to others in the literature. Lustmann J and Bodner L reported on DC associated with SN teeth [21]. In a review of 42 such cases from their own material and those reported in the literature, they found that about 90% were associated with a maxillary mesiodens. In the present study too, 60% of cases were associated with mesiodens (6/10, 60%).
Radiographically, DC present as unilocular radiolucency around the crown of an unerupted tooth with sclerotic border, which was evident in 48 (70.58%) cases in the present study [4]. However, few cases which presented as multilocular radiolucency (13.23%) could be due to pseudotrabeculation as reported by Shear M [4]. Root resorption of adjacent teeth, though common in DC, was not a significant finding in the present study (7.35%) compared to other studies in the literature like Struthers PJ and Shear M who observed root resorption in 11 of 20 DC (55%) [22]. They suggested that the potential of DC for root resorption may be derived from its origin from dental follicle [22]. DC may often be associated with other pathology especially other odontogenic cysts and odontogenic tumours. DC, in our series also showed association with OKC, radicular cyst, AOT and an odontome in separate cases. Diagnosis of DC is straight forward when it is associated with an unerupted tooth. However, not all lesion associated with unerupted teeth are DC. Unerupted tooth may be seen associated with other odontogenic lesions like KCOT, ameloblastoma, especially unicystic ameloblastoma and AOT. Conventional radiographs and CBCT may play an important role in differentiating these lesions from DC.
The 61.76% of cases in the present study were provisionally diagnosed as DC based on clinical and radiological grounds. The remaining cases, (33.82%) which were clinically diagnosed as radicular cyst (7, 10.29%), unicystic ameloblastoma (11, 16.17%) and AOT (5, 7.35%) were diagnosed as DC on histopathological examination contrary to the clinical diagnosis. Histopathological appearance of DC was similar to various reports and showed a thin non-keratinised stratified squamous to cuboidal epithelial lining resembling reduced enamel epithelium [4]. Considerable variation in the nature of the epithelial lining of cysts have been observed. Occasionally, DC may show diverse histopathological finding like proliferation, keratinisation and metaplastic changes into mucous, sebaceous, squamous and ciliated cells in the lining [23-25]. These findings supports the pleuripotential nature of odontogenic tissue. Although, keratinisation is rare in DC, it has been reported in literature. Yeo JF et al., reported keratinisation in DC lining, being either parakeratinisation or orthokeratinisation [8]. The present series also showed lining undergoing parakeratinisation (3) which could be attributed to inflammation in few cases.
Neoplastic transformation of DC lining is not an uncommon finding. Both benign and malignant tumours arise from DC lining. Various tumours like ameloblastoma, AOT, squamous cell carcinoma and CEOT have been known to originate from DC lining [2,4,5,26]. The present series showed, AOT arising from DC in 9 (11.6%) cases. This association concurs with various studies in the literature. Only 19 articles were registered in the International literature (Pubmed) from 1960-2016, connecting AOT with DC [27,28].
The histogenesis of AOT is controversial with various concepts being proposed as to its origin from fully formed enamel organ, dental lamina and/or its remnants and occasionally from odontogenic cysts [4,5]. There is considerable controversy as to whether the cystic component of AOT has been considered as either a true DC or cystic change in the AOT or it may represent a distinct hybrid variant [28]. Some authors believe that the cyst develops first and then the AOT arises from the epithelial rests of dental lamina within the odontogenic cyst lining due to some unknown stimulus [5,29].
Limitation
Further studies including large series of DC should be performed in different regions of the world in order to determine the global epidemiologic profile of these lesions as this study is limited to a certain local population of district of North interior, Karnataka, India.
Conclusion
This study provides a data on DC in North Karnataka region of India. Based on findings in the present study, it can be concluded that the changes within the epithelium of a DC should carefully be recognised by the pathologist while reporting the biopsy as it is important to distingush between unicystic ameloblastoma, AOT and a rare potential malignant transformation of the cyst.