Breast carcinoma is the most common malignant tumour worldwide and the leading cause of death in women [1]. In India, breast cancer is the most common cancer among women followed by cervical cancer [2]. The functional unit of the breast is the single gland, arranged into lobes and made-up of two major components: the Terminal Duct Lobular Unit (TDLU) and the large duct system [1]. The epithelium is bilayered, an inner epithelial layer and an outer myoepithelial layer. Myoepithelial cell layer is one of the main guide to the distinction between benign and malignant lesions [3]. Most of the breast malignancies are adenocarcinomas and based on the expression of Estrogen Receptor (ER), Progesterone Receptor (PR) and HER2 they can be divided into four major subgroups: luminal A, luminal B, HER2-overexpressing, and basal-like [4]. Immunohistochemical classification also provides both therapeutic and prognostic information. Antiestrogen (tamoxifen) nowadays become treatment of choice in ER positive case [5].
The basal-like subtype, characterised by negativity for ER, PR and HER2, is associated with aggressive histology, poor prognosis, and unresponsiveness to the usual endocrine therapies [6]. HER2/neu is a member of the ErbB family that plays an important role in promoting estrogen transformation and tumour growth [7]. HER2 positivity is also useful for targeted therapy with monoclonal antibody (trastuzumab) against HER2 [8]. The objective of present study was to know the association of molecular classification with clinicopathological parameters for prognostic significance in breast cancer. The present study aim was also to follow up the patients with recurrence in regard to tumour mass, lymph node and surgical margin status and changes in IHC pattern.
Materials and Methods
This prospective study was conducted in the Department of Pathology in association with Department of General Surgery in Institute of Post Graduate Medical Education and Research, Kolkata, West Bengal, India (from January 2014 to June 2015) after taking Ethical Committee approval. Total 50 patients presenting with palpable breast lump undergoing mastectomy whose clinical symptoms were suggestive of breast cancer and patients having recurrence undergoing further treatment at Institute of Post Graduate Medical Education and Research were included.
The specimens were collected and further studies were done in Department of Pathology. H&E staining was done to see the histological features and IHC staining was done for expression of ER, PR and HER2 [Table/Fig-1,2 and 3].
Estrogen receptor positivity in patient of Invasive Ductal Carcinoma, No Special Type (IDCNST), (IHC staining, 40X).
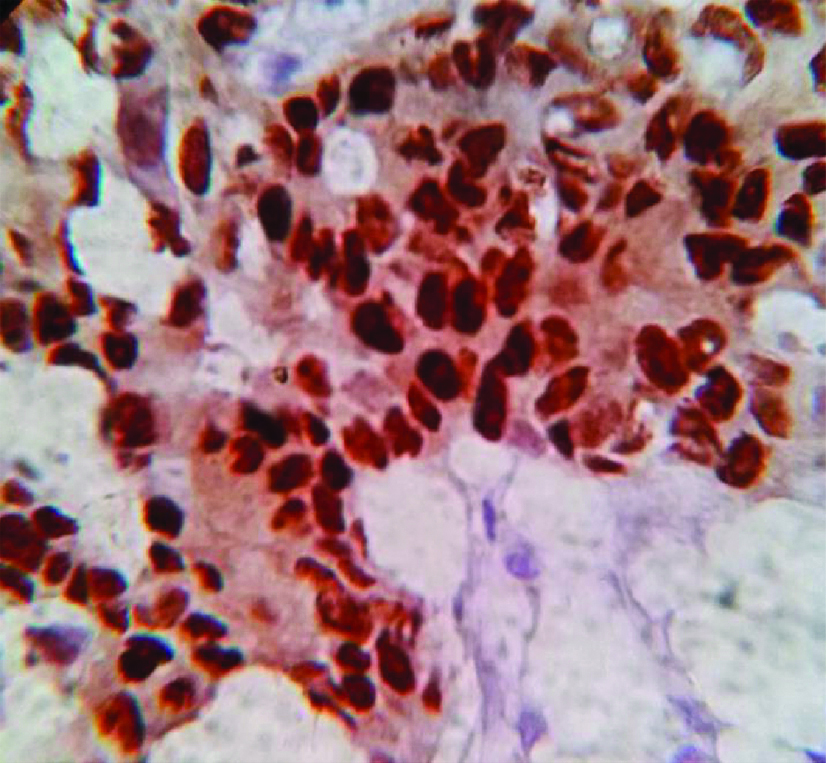
Photomicrograph showing progesterone receptor positivity in a patient of in patient of invasive ductal carcinoma, no special type, (IHC staining, 40X).
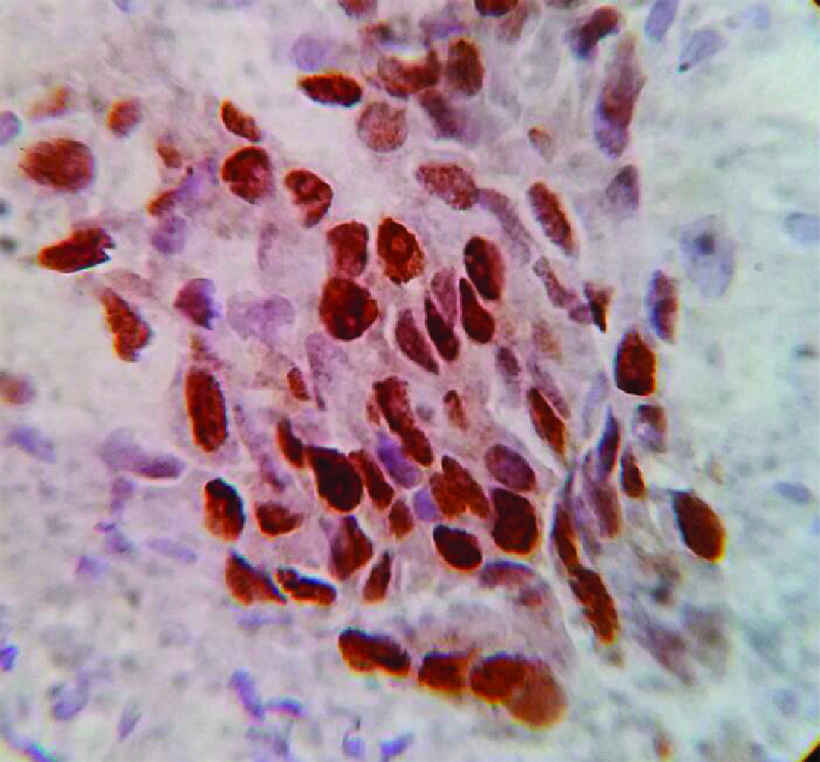
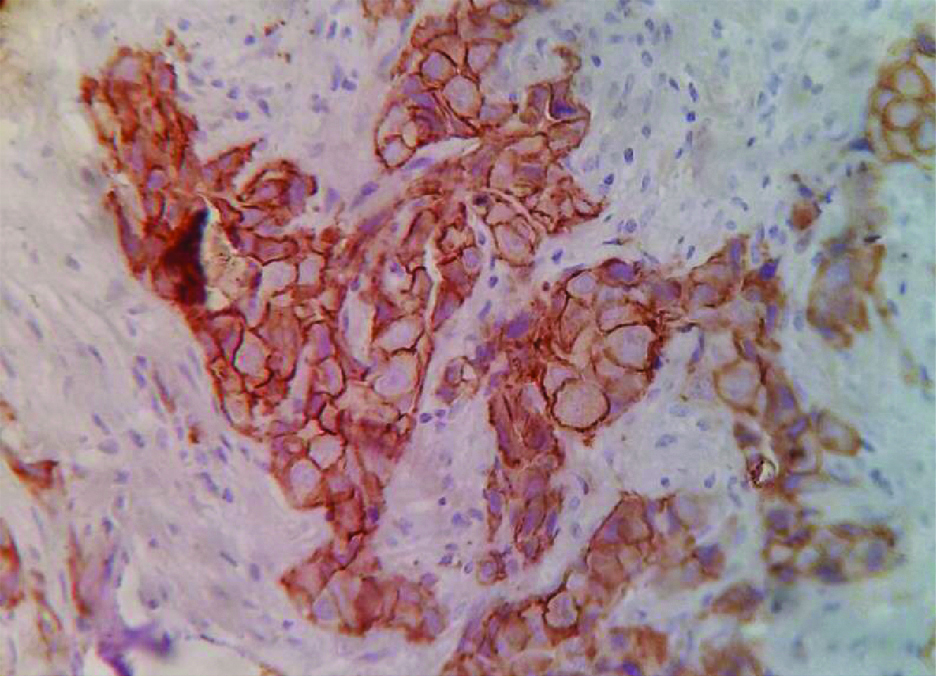
Photomicrograph showing: Human Epidermal Growth Factor 2 (HER2)/neu positivity, (IHC staining 40X).
The clinicopathological features such as tumour size, histological type, nottingham combined histologic grade, lymphovascular invasion, perineural invasion, status of margins, associated in situ component/Paget’s disease and lymph nodal status were assessed.
They were classified according to IHC staining into five molecular subtypes: Luminal A-like, luminal B-like HER2 (-), luminal B-like HER2(+), HER2+ and triple negative/basal like. IHC study was also done on recurrent masses following surgical removal.
Interpretation of Immunostaining
a. ER/PR: All cases with at least 1% of positive cells were considered positive and quantification was done using Allred scoring system [9,10].
b. HER2: Interpretation was done according to American Society of Clinical Oncology (ASCO)/College of American Pathologist (CAP) Guidelines [11].
Definition of Molecular Subtypes
Molecular subtypes were defined depending upon IHC expression of ER, PR, and HER2 and according to St. Gallen 2013 consensus conference in the following ways [Table/Fig-4] [12].
Definition of molecular subtypes according to immunohistochemical expression.
| Molecular Subtype by IHC-surrogate | Criteria |
|---|
| Luminal A-like | All of ER and PR positive, HER2 negative, Ki-67 low |
| Luminal B-like (HER2 positive) | ER positive HER2 overexpressed any Ki-67 any PR |
| HER2 overexpression | HER2 positive (non-luminal) HER2 over-expressed or amplified ER and PR absent |
| Basal like | Triple negative (ductal)’ ER and PR absent HER2 negative |
| PPN | Luminal A | 25 |
| PPP | Luminal B | 9 |
| NNP | HER2/neu | 11 |
| NNN | Basal like | 5 |
P: Positive symbolise, N: Negative symbolise, HER2: Human Epidermal Growth Factor 2, ER: Estrogen Receptor, PR: Progesterone Receptor
Statistical Analysis
All statistical tests were performed and analysed by the software IBM SPSS statistics version 20.0. Statistical significance was determined at p<0.05. The chi-square test was conducted to assess the relationship between categorical variables.
Results
Age of participants ranged from 35-70 years with median age being 54.6 years. Breast carcinoma was most commonly found in the age group of 46-55 years (22 cases) followed by 56-65 years (16 cases), 35-45 years (nine cases) and >65 years (three cases). All the patients were female. Among all the cases 24 involved the right breast and 26 involved the left breast. So, apparently left breast involvement was slightly higher than the right (52% versus 48%). Upper outer quadrant was most commonly involved 29 cases (58%) followed by lower outer quadrant (10 cases), upper inner quadrant (eight cases) and lower inner quadrant (three cases.)
Different sizes of tumour were noticed starting from 1.2 cm to 6.2 cm. According to TNM staging, maximum tumour masses were in the T2 group 25 (50%) followed by T3 (12), T1(8) and T4 (5). Surgical margin was involved only in five cases whereas, in rest 45 surgical margin was not involved. Only two patients showed metastasis. Among the 50 patients six patients had history of breast carcinoma among first degree relatives.
Among the 50 patients, 43 (86%) were suffering from Invasive Ductal Carcinoma, No Special Type (IDCNST) followed by Infiltrating Lobular Carcinoma (ILC) (three cases), metaplastic carcinoma and medullary carcinoma (each two cases). Histological grading was done on all the specimens Nottingham Histological Grading System (NHS) Among the 50 patients, 26 had Grade I tumour (52%),14 had Grade II tumour and 10 had Grade III tumour.
Subsequently, IHC study (ER, PR and HER2/neu) was done on all the cases and they were accordingly classified. Maximum cases (25) were luminal A subtypes (ER-positive, PR-positive, HER2-negative). Correlation between histological grade and IHC subtype is shown in [Table/Fig-5]. High grade tumours were generally HER2/neu or basal subtype. The correlation between histological subtype and IHC subtype is given in [Table/Fig-6].
Correlation between histological grade and IHC subtypes.
| IHC subtypes | NHS Gr1 | NHS Gr2 | NHS Gr3 |
|---|
| Luminal A | 21 | 4 | 0 |
| Luminal B | 0 | 6 | 3 |
| HER2/neu | 0 | 5 | 6 |
| Basal | 0 | 0 | 5 |
p-value <0.0001
NHS: Nottingham Histological Grading System, Gr: Grading, HER2/neu: Human Epidermal Growth Factor 2
Correlation between histological type and IHC subtypes.
| IHC subtypes | Medullary | Metaplastic | IDCNST | ILC |
|---|
| Luminal A | 0 | 0 | 22 | 3 |
| Luminal B | 0 | 0 | 9 | 0 |
| HER2/neu | 0 | 0 | 11 | 0 |
| Basal | 2 | 2 | 1 | 0 |
IDCNST: Invasive Ductal Carcinoma, No Special Type, ILC: Infiltrating Lobular Carcinoma, HER2/neu: Human Epidermal Growth Factor 2
Correlation between tumour size and IHC subtypes is given in [Table/Fig-7]. The correlation between lymph node status and IHC pattern is presented in [Table/Fig-8].
Correlation between tumour size and IHC subtypes.
| IHC subtypes | Luminal A | Luminal B | HER2/neu | Basal |
|---|
| T1 | 6 | 1 | 1 | 0 |
| T2 | 16 | 4 | 4 | 1 |
| T3 | 2 | 3 | 4 | 3 |
| T4 | 1 | 1 | 2 | 1 |
p-value=0.28
HER2/neu: Human Epidermal Growth Factor 2, IHC: Immunohistochemistry
Correlation between lymph node status and IHC subtype.
| Luminal A | Luminal B | HER2/neu | Basal |
|---|
| N0 | 8 | 1 | 1 | 0 |
| N1 | 8 | 4 | 1 | 1 |
| N2 | 5 | 4 | 7 | 3 |
| N3 | 4 | 0 | 2 | 1 |
p-value is 0.03
HER2/neu: Human Epidermal Growth Factor 2
Now regarding recurrence the study showed that among 50 patients only five patients developed recurrent mass. Patient with initial T1 mass showed no recurrence. Among the 24 patients with initial T2 mass one patient developed recurrent mass, three patients out of nine with T3 mass suffered from recurrence whereas, one patient out of four with T4 mass developed recurrence (p-value=0.07).
The node status among the five patients who developed recurrence had initial lymph node status was as follows: N0-0 patient, N1-1 patient, N2-3 patients and N3-1 patient. After the study period it is found that, three patients in N0 status, two patients in N1 status and no patient found in N2 or N3 status.
Subsequently, the recurrent masses were surgically removed. IHC study was done with ER, PR and HER2/neu. Five patients who developed recurrence, their initial IHC study showed that, two patients were HER2/neu subtype and three patients were triple negative. However, after reoperation it was found that all the patients have become triple negative. Five patients who had recurrence all were initially grade III (p-value=0.0007). Surgical margin was involved in five patients of which two patients developed recurrent mass, p-value was 0.02. Among the 50 patients six patients had Pagets disease. Two of them had grade II disease and four had grade III disease. No grade I patient had Pagets disease.
Discussion
The present study was a single institution based study on mastectomy specimens of breast carcinoma. ER, PR and HER2/neu were all carried out by a single laboratory and evaluated by the same core group of pathologists, thus ensuring the reproducibility of results. In the present study total 50 patients were included (all female). Khemka A et al., expressed that the peak incidence of breast carcinoma was between 40-44 years in their study [13]. Of the 50 patients 26 (52%) patients had breast carcinoma involving left side. Aljarrah AL and Miller WR stated that breast carcinoma involves both breast almost equally [14]. Present finding slightly differs with them.
Among all the 50 patients 29 (58%) patients showed involvement by breast carcinoma in right upper quadrant followed by lower outer quadrant (20%) and then other quadrants. Hussain MT et al., in his series had 58% of patients in whom upper outer quadrant was involved in breast lumps [15]. Tumour location has an important role in the breast cancer prognosis because from previous studies it has been seen that tumours situated in central/internal quadrants have a worse prognosis than to those in lateral quadrants, in relation to distant metastasis and survival [16].
In the present study, size of tumour masses was found starting from 1.2 cm to 6.2 cm. Most commonly patients were having T2 tumour 25 (50%) patients, followed by T3 (24%), followed by T1 and T4. The size of a mammary carcinoma is one of the most significant prognostic variables. Ahmed Z et al., showed that 44.16% patients were having T2 tumour followed by T3 (41.16%), T1 and T4 [17]. So, the result of present study was more or less same as them.
Of the 50 patients, 43 (86%) patients were suffering from IDCNST followed by ILC (6%), medullary carcinoma and metaplastic carcinoma. Eheman CR et al., showed in their study that around 70% patients were suffering from IDCNST with ductal carcinoma in situ [18]. The present study result slightly differs from their results, it may be due to a smaller study population in present study.
Now coming to the IHC studies, 25 (50%) of 50 patients were of luminal B subtype followed by HER2/neu subtype (22%), luminal B (18%) and basal type (10%). The prevalence of triple negative group in the present study (10%) was similar to that in European populations (10-16%), but slightly lower than in African-American population (20-21%) [19]. Verma S et al., also reported similar findings but the study by Munjal R et al., found higher percentages (22.5%) [20,21]. The HER2 subtype accounted for 22% of cases, which is higher than the reported positivity (4-8%) in either European or African-American populations [19,22]. In the present study, women with triple-negative breast cancer had more frequent association with family history than with other subtypes. This suggests that genetic factors may play an important role in this molecular subtype of breast cancer. The finding of higher HER2 positive tumours is also a common finding in Chinese population based study [23]. This may suggest a distinct biologic property of Asian breast carcinoma, further supporting the heterogeneous nature of breast carcinoma.
All NHS grade I patients were belonging to luminal A subtype. Among the NHS grade II, four were luminal A type, six were luminal B type and five were HER2/neu subtype. Among the NHS grade III three were luminal B subtype, six were HER2/neu subtype and five were basal type. Bhagat Vasudha M et al., in their study showed that Grade I tumour showed ER, PR positivity was more as compared to Grade III tumours. HER-2/neu expression and ER, PR receptor status showed inverse relationship. HER-2/neu expression was more with size and high grade of tumour [24]. So, the present result also corroborates with them.
From the present study, we also came to know that, patients with initial T1 tumour showed no recurrence, whereas one in 25 patients with initial T2 tumour, three in 12 patients with initial T3 tumour and one in five patients with initial T4 tumour showed recurrence. Similarly, it was also found that the patients who suffered from recurrence, all had grade III tumour initially. Cornfield DB et al., also showed in their study that the histological grade of the tumour and chances of recurrence is highly proportional to each other [25].
Among the five patients who had recurrence, two were HER2/neu type and three were basal type initially. After surgical removal of the recurrent mass IHC study was done again and it was found that all the patients have now became triple negative (basal type). Ontilio AA et al., also showed that the triple negative subtype has the worst overall and disease free survival [26].
Limitation
As it is a single institute based study and the study period was very short, the number of cases were small. Longer prospective studies are required to better assess the prognosis and appearance of recurrent mass. The main weaknessis of IHC approaches are limited technical reproducibility, subjective interpretation, and qualitative readouts although, all the IHC was performed and interpreted in a single laboratory by same core group of pathologists to avoid these issues as much as possible.
Conclusion
From the present study, we came to a conclusion that, most common breast carcinoma is IDCNST whereas, most of the patients were suffering from NHS grade I. Regarding IHC study, the present study clearly reveals that almost all of the low grade tumours were luminal A subtype. As the gradation of the tumour increases their IHC pattern also changed. HER2/neu expression also changed remarkably with gradation. So, from above discussion it is clear that luminal A and luminal B subtype have relatively favourable prognosis whereas, HER2/neu and basal subtype have most poor prognosis. These IHC subtypes are also proportionate with NHS grading. The patients with higher NHS grades were either HER2/neu or basal like subtype. From the follow up study we also found that all the patients who had recurrence initially were either HER2/neu and basal subtype.
Larger, population based trial is required to delineate prevalence, clinicopathological features and prognosis of the patients and longer follow up period is required for studying the recurrence. The reliability and accuracy of the tests can be increased by arranging skilled courses and proper training for the technical staff. Fluorescence In Situ Hybridisation (FISH) or Chromogenic In Situ Hybridisation (CISH) techniques for evaluation of HER2 positive cases are preferred to IHC where available.
p-value <0.0001
NHS: Nottingham Histological Grading System, Gr: Grading, HER2/neu: Human Epidermal Growth Factor 2
IDCNST: Invasive Ductal Carcinoma, No Special Type, ILC: Infiltrating Lobular Carcinoma, HER2/neu: Human Epidermal Growth Factor 2
p-value=0.28
HER2/neu: Human Epidermal Growth Factor 2, IHC: Immunohistochemistry
p-value is 0.03
HER2/neu: Human Epidermal Growth Factor 2