Gestational Diabetes Mellitus, one of the most common metabolic disorders of pregnancy, has been defined by the World Health Organisation (WHO) as glucose intolerance resulting in hyperglycaemia of variable severity, with onset or first recognition during pregnancy [1]. The prevalence of GDM is variable and depends on the population, race and the diagnostic criteria defined by each country. American Diabetes Association(ADA) estimated that some 7% of all pregnancies are affected by GDM while according to National Guidelines by Ministry of Health and Family Welfare prevalence rate of GDM in India was estimated to be 10-14.3% and it was 17.8%, 13.8% and 9.9% in the urban, semi urban and rural areas respectively [2,3]. Bortolon LMN et al., found higher prevalence of GDM among Asian, Latin American and Indian women [4]. GDM is an important public health concern as its prevalence is increasing steadily due to advanced maternal age, increasing urbanisation, and obesity epidemic.
Pregnancies with GDM are associated with increased incidence of adverse maternal outcome such as pregnancy induced hypertension, polyhydramnios, caesarean section and higher risk of developing type 2 diabetes in future compared to pregnancies without GDM [5]. The risk of perinatal mortality per se is not increased but morbidity due to the risk of macrosomia, shoulder dystocia, birth injuries such as bone fractures, nerve palsies and hypoglycaemia is increased. Long term risks among such infants include impairment of glucose tolerance, obesity, impaired intellectual achievement, cardiovascular disease [6].
Screening of GDM is important because women who are at high risk for developing the disease can be identified and the risks to maternal and foetal health can further be reduced. The debate on GDM screening, diagnosis and timing of screening still persists in the various professional societies all over the world as different criteria are used for its screening and diagnosis. In 2010, based on the results of Hyperglycaemia and Pregnancy Outcome (HAPO) and other studies, the International Association of Diabetes and Pregnancy Study Groups (IADPSG) issued a consensus statement on new criteria for the diagnosis and screening of GDM so that the screening of GDM may be initiated during the first prenatal consultation [7]. FPG is a useful test for screening gestational diabetes mellitus and has been recently compared with other measures of hyperglycaemia for diagnosing gestational diabetes because of its simplicity, low cost, reproducibility and worldwide availability [8]. However, very few studies of FPG as a screening test for GDM are available in India [9,10], so this study was undertaken with the objective to find out the predictive value of FPG on first antenatal visit before 20 weeks of gestation for the diagnosis of GDM and to correlate it with OGTT with 75 gm glucose at 24-28 weeks of gestation.
Materials and Methods
It was a hospital based prospective study which was conducted on antenatal patients in the Department of Obstetrics and Gynaecology at Hindu Rao Hospital, Delhi, India, from June 2014 to May 2016 in close association with the Department of Biochemistry. It was approved by the Ethics Committee. This study included 256 pregnant women attending the antenatal clinic who had gestation less than 20 weeks. Ten patients were lost in follow up so, 246 patients were studied. Pregnant women after 20 weeks of gestation and with history of preexisting diabetes or with fasting glucose >126 mg% at first antenatal visit were excluded from the study as they were overt diabetics.
Patients were enrolled in the study only when an informed written consent was obtained. Pre structured Performa was used to record the details of the patients including age, demographic profile, obstetric history, menstrual history, past history and family history. Any history of GDM in the past and any family history of diabetes were noted. General physical examination and systemic examination of the patients was done and BMI was calculated. Routine investigations like complete blood count, blood group and Rh factor, HIV, HBsAg and VDRL, serum TSH, urine (routine and microscopic examination) and routine ultrasound were done.
The patients were subjected to FPG test on first antenatal visit. GDM was diagnosed when FPG was ≥92 mg/dL but < 126 mg/dL. This was in accordance with IADPSG criteria which states that: [7]
Overt diabetes is classified if any of the following are found at the first antenatal visit.
FPG >126 mg/dL, OR
HbA1c ≥6.5% OR
Random plasma glucose ≥200 mg/dL plus confirmation with FPG or HbA1c.
All the enrolled patients were subjected to OGTT with 75 gm of glucose in the morning after an overnight fasting of about eight hours from 24 to 28 weeks of gestation. In the patients with fasting glucose ≥92 mg/dL and <126 mg/dL, no intervention was done till they were subjected to OGTT at 24-28 weeks of gestation. Diagnostic criteria for GDM were in accordance with IADPSG criteria and it was diagnosed when one or more values of plasma glucose exceeded threshold levels at 24-28 weeks of gestation [7].
Fasting plasma glucose (0 hour) ≥92 mg/dL.
One hour ≥180 mg/dL.
Two hour ≥153 mg/dL.
Cutoff levels of fasting glucose were analysed as screening test with the result of OGTT as the standard diagnosis of GDM. Patients were regularly followed up in antenatal OPD till delivery and maternal and foetal outcome was recorded.
Statistical Analysis
Statistical analysis of the test was done and the outcome was measured by Pearson Chi-square test. The Unpaired t-test was used to compare the discrete variables between GDM and NGT. The Pearson correlation analysis was carried out to find the correlation between two discrete variables. The ROC analysis was performed to find the cutoff value of FPG as well as sensitivity and specificity to diagnose GDM. The Area Under the Curve (AUC) with its 95% Confidence Interval (CI) was also calculated. All the analysis was carried out on SPSS software version 16.0 (Chicago, Inc., USA). The p-value of ≤0.05 was considered statistically significant.
Results
In the study out of 246 patients, 22 patients had FPG ≥92 mg/dL but <126 mg/dL. Out of these 22 patients, 16 had abnormal OGTT at 24-28 weeks of gestation and were diagnosed as GDM. Rest of the 230 patients (including six patients who had raised fasting glucose at first antenatal visit) had NGT. As shown in [Table/Fig-1], 50% of the women with GDM were in the age group 26-30 years while, 21.3% of NGT were between 26-30 years. There were 68.8% primi gravida and 31.3% multi gravida in the GDM group while in the NGT group, 37% were primi and 63% were multi gravida. Majority of the women in both GDM (87.5%) and NGT (62.2%) groups had no past history of abortions. None of the women of GDM group or NGT group had past history of GDM. 4.8% of the NGT group had family history of diabetes and none of the patients in GDM group had family history of diabetes.
Distribution of the women between GDM and NGT groups according to age, gravidity, outcome of previous pregnancy and presence of glycosuria.
| Age (years) | GDM | NGT | p-value1 |
|---|
| No. | % | No. | % |
|---|
| 20-25 | 8 | 50.0 | 147 | 63.9 | 0.01* |
| 26-30 | 8 | 50.0 | 49 | 21.3 |
| >30 | 0 | 0.0 | 34 | 14.8 |
| Gravidity |
| One | 11 | 68.8 | 85 | 37.0 | 0.07 |
| Two | 3 | 18.8 | 63 | 27.4 |
| Three | 2 | 12.5 | 61 | 26.5 |
| Four | 0 | 0.0 | 21 | 9.1 |
| Urine sugar |
| Present | 5 | 31.2 | 0 | 0 | 0.001* |
| Absent | 11 | 68.8 | 230 | 100.0 |
1Chi-square test, *Significant, GDM-Gestational Diabetes Mellitus, NGT-Normal Glucose Tolerance
The [Table/Fig-2] shows the mean of FPG and levels of OGTT at 0, one, two hour in GDM and NGT groups. The [Table/Fig-3] shows that 62.5% cases of GDM underwent Lower Segment Caesarean Section (LSCS) as compared to 26.1% of the NGT group. In the present study, out of 16 cases of GDM 37.4% babies weighed <3 kg and 56.2% weighed between 3.0-3.99 kg while 6.2% of cases had birth weight ≥4 kg. In the NGT group, 77.8% of cases had birth weight <3 kg while 22.2% of cases had birth weight ranging from 3-3.49 kg. No baby in the NGT group was more than 3.5 kg.
Mean FPG at first visit at <20 weeks of gestation and Mean OGTT at 24-28 weeks gestational age among GDM and NGT groups.
| FPG (Mean±SD) | OGTT (Mean±SD) |
|---|
| At 0 hour | At one hour | At two hour |
|---|
| GDM (n=16) | 99.44±10.26 | 106.31±10.66 | 187.75±4.21 | 157.00±13.96 |
| NGT (n=230) | 76.26±10.35 | 76.00±7.45 | 156.31±9.26 | 129.66±10.73 |
| p-value1 | 0.0001* | 0.0001* | 0.0001* | 0.0001* |
1Unpaired t-test, *Significant, FPG-Fasting Plasma Glucose GDM-Gestational Diabetes Mellitus, NGT-Normal Glucose Tolerance
Distribution of the women between GDM and NGT according to mode of delivery and foetal weight.
| Maternal outcome (Mode of delivery) | GDM | NGT | p-value1 |
|---|
| No. | % | No. | % |
|---|
| Normal | 6 | 37.5 | 170 | 73.9 | 0.002* |
| LSCS | 10 | 62.5 | 60 | 26.1 |
| Foetal outcome weight in kg |
| <2.5 | 1 | 6.2 | 44 | 19.1 | 0.001* |
| 2.5-2.99 | 5 | 31.2 | 135 | 58.7 |
| 3.0-3.49 | 4 | 25.0 | 51 | 22.2 |
| 3.5-3.99 | 5 | 31.2 | 0 | 0.0 |
| ≥4.0 | 1 | 6.2 | 0 | 0.0 |
| Mean±SD | 3.16±0.52 | 2.71±0.29 | |
1Chi-square test, *Significant, GDM-Gestational Diabetes Mellitus, NGT-Normal Glucose Tolerance
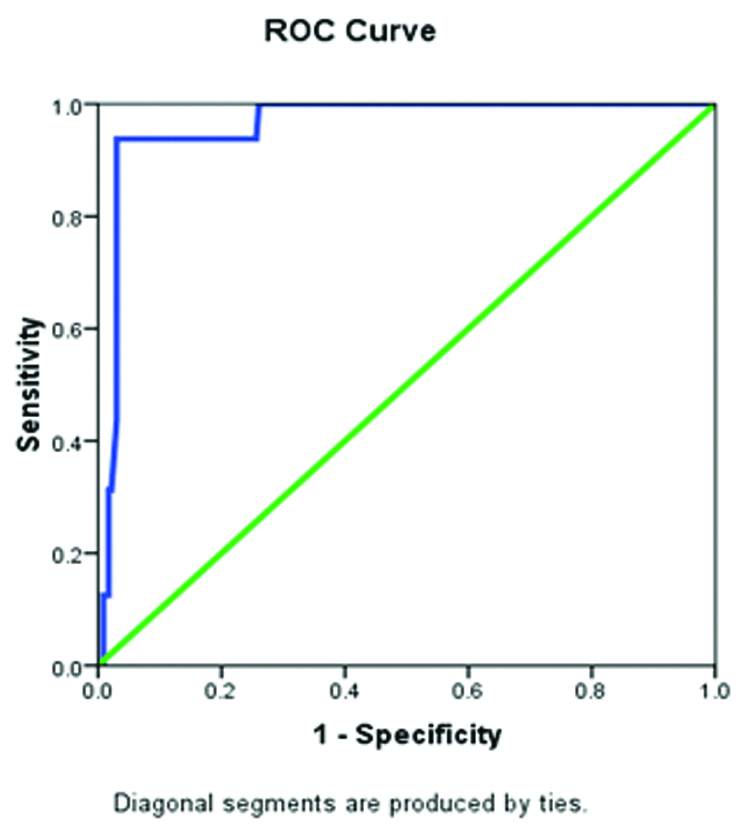
Correlation coefficient (r) of FPG in pregnant women with <20 weeks gestation at the first visit and OGTT at 0, one, two hours in the GDM group was 0.88, 0.64 and 0.48 respectively while it was 0.60, 0.19 and 0.15 respectively in the NGT group [Table/Fig-4]. There was a strong positive correlation between FPG and OGTT at 0, one and two hours with p-value 0.001, 0.001 and 0.005 respectively. A cut off value of 84.5 mg/dL has the highest sensitivity (93.8%) and specificity (74.3%) [Table/Fig-5]. The sensitivity and specificity of FPG at various cutoffs are drawn on ROC curve [Table/Fig-6].
Correlation of FPG at < 20 weeks of GA and OGTT at 24-28 weeks of gestation.
| OGTT | Correlation coefficient (r) with FPG1, p-value |
|---|
| GDM | NGT | Total |
|---|
| At 0 hour | 0.88, 0.001* | 0.60, 0.001* | 0.73, 0.001* |
| At one hour | 0.64, 0.007* | 0.19, 0.003* | 0.25, 0.001* |
| At two hour | 0.48, 0.06 | 0.15, 0.02* | 0.18, 0.005* |
1Pearson correlation analysis, *Significant, GDM-Gestational Diabetes Mellitus, OGTT-Oral Glucose Tolerance Test, NGT-Normal Glucose Tolerance
Predictive value of FPG at cut off level > 84.5 mg/dl for diagnosis of GDM.
| Cut off value of FPG (mg/dl) | | GDM +ve | GDM -ve | Predictive value of FPG |
|---|
| | | Sensitivity | Specificity | PPV | NPV | LR+ve | LR -ve | AUC (95%CI), p-value |
|---|
| ≥84.5 | FPG +ve | 15 | 59 | 93.8 | 74.3 | 20.3 | 99.4 | 3.7 | 0.08 | 0.96 (0.92-0.99), 0.0001* |
| FPG-ve | 1 | 171 |
LR- Likelihood ratio, AUC- Area under the curve. The AUC (95% CI) was 0.96 (0.92-0.99) with p=0.0001, PPV-Positive Predictive Value, NPV-Negative Predictive Value
FPG-Fasting Plasma Glucose, GDM-Gestational Diabetes Mellitus
ROC showing the sensitivity and specificity of FPG to diagnose GDM.
ROC-Receiving operating curve, FPG-Fasting Plasma Glucose, GDM-Gestational Diabetes Mellitus
Discussion
The mean gestational age among the enrolled pregnant women in our study on first antenatal visit was 16.48±1.77 weeks. The mean age of the patients in the GDM and NGT group was 24.56±2.87 and 25.11±4.11 year respectively. More patients were in the older age group (26-30 years) in the GDM group compared to NGT group. The difference was statistically significant (p=0.01). In comparison Gracelyn LJ et al., observed the mean age among GDM women was 28.47±3.38 years and found that the correlation between age and GDM was statistically significant [11]. Kalyani KR et al., observed mean age of 24.16±3.63 years in their study and found that gestational diabetes was more common in women above 25 years of age [12]. There was no statistically significant difference in the obstetrical history and outcome of previous pregnancy between GDM and NGT group (p-value=0.07) and (p-value=0.11) respectively. In comparison Seshiah V et al., found that the prevalence of GDM is rising with gravidity, from 18.1% in first pregnancy to 25.8% in grand multi para but Verghese R et al., observed no statistical significance in parity [13,14].
In our study, the risk factors like past history and family history of GDM had no statistical significance. In comparison Chong YS et al., observed that previous history of GDM had significantly increased risk in Chinese and Malaysian women [15]. Gracelyn LJ and Sarayana N, observed that 18.64% of GDM women had GDM in their previous pregnancy with Odds Ratio or 8.95 and found a significant association between history of GDM in previous pregnancy and occurrence of GDM in the index pregnancy. They also, observed a strong correlation between the family history of diabetes with the development of GDM in pregnancy [11]. Our study had included patients from low socioeconomic class, so due to ignorance and lack of education they were not able to give any past history of GDM and family history of diabetes mellitus.
The mean BMI was 22.97±2.68 kg/m2 in the GDM group and 23.25±2.59 kg/m2 in the NGT group. There was no statistically significant difference in BMI between GDM and NGT group in our study because the patients were from poor socioeconomic status and there was lack of proper nutrition so obesity was uncommon. In comparison the mean BMI in the GDM group was 26.07±4.44 kg/m2 in the study by Gracelyn LJ and Sarayana N, and they noted statistically significant positive correlation between GDM and obesity [11].
Women with GDM are at high risk of developing hypertensive disorders such as Gestational hypertension, preeclampsia and eclampsia. In our study as the pregnancy advanced, three cases (18.75%) in the GDM group and 12 cases (5.2%) in the NGT group developed preeclampsia during pregnancy. There was a relative risk of 3.6 for development of preeclampsia in GDM. In Comparison Verghese R et al., observed that the gestational hypertension occurred in 14.4% of patients with GDM [14] while Mardi TG et al., observed that there was a significant association between GDM and development of proteinuria with a relative risk of 1.98 [16].
Glycosuria was observed in 31.2% in GDM group and none in the NGT group and it was statistically significant (p-value=0.001). Glycosuria among pregnant women is observed due to relatively low glomerular filtration rate and low renal threshold for glucose. In comparison Mardi TG et al., observed a strong association between glycosuria and development of GDM with a relative risk of 2.36 [16] while our study showed a relative risk of 2.1 for the development of GDM in presence of glycosuria.
The mean of FPG in GDM cases was 99.44±10.26 mg/dL and in NGT group it was 76.26±10.35 mg/dL and the difference was statistically significant (p=0.0001). In comparison the mean FPG values of women with GDM was 103.85±14.93 mg/dL compared to 86.22±6.70 mg/dL in normal women by Rajput R et al., (p-value <0.001) [17]. Mean second hour OGTT levels in the GDM and NGT group were 157.00±13.96 and 129.66±10.73 respectively in our study (statistically significant p-value=0.0001) while Palur H et al., observed the mean second hr blood glucose values of 154.32±8.7 vs. 98±14 mg/dL in GDM and non-GDM cases respectively [18].
Caesarean section rate was 62.5% in the GDM group with most common indication of cephalopelvic disproportion due to good size baby while in the NGT group, it was foetal distress and previous LSCS. Difference in the mode of delivery in both the groups was statistically significant (p-value=0.002). In comparison Verghese R et al., reported caesarean delivery in 92.80% of women with GDM and found mode of delivery statistically significant [14] while Riskin-Mashiah S et al., observed that the primary caesarean section rate increased from 12.7 to 20.0% [19].
The mean of the foetal weight in NGT and GDM group was 2.71±0.29 kg and 3.16±0.52 kg respectively. None of the cases in the NGT group had birth weight more than 3.5 kg [Table/Fig-4]. Difference in the foetal weight in both the groups was statistically significant (p-value= 0.001). 37.4% of cases in the GDM group had good size baby (>3.5 kg). Birth weight more than 3.5 kg does not fulfill the criteria of macrosomia (implies foetal growth beyond specific weight, usually 4kg or 4.5kg, regardless of foetal gestation age) [20]. However, the patients included in our study are from poor socioeconomic status and had short height and low BMI, the birth weight >3.5 kg may be considered as macrosomia for this ethnic group of patients. Caesarean section was done in all the cases of GDM group with foetal birth weight >3.5 kg. No birth injury was detected in our study. Five babies (31.25%) in the GDM group with birth weight >3.5kg were admitted in neonatal ICU whereas in the NGT group 12 babies (5.2%) were admitted in neonatal intensive care unit due to various reasons such as foetal distress, delayed cry, meconium stained liquor. In comparison Robin V et al., observed no macrosomia in their study [14] while Riskin-Mashiah S et al., observed that the frequency of Large for Gestational Age (LGA) neonates and or macrosomia increased from 7.9 to 19.4% [19]. In comparison Kalyani KR et al., observed that 56% women belonging to GDM group had their babies admitted to NICU as compared to only 21.33% women of the non GDM group with babies requiring NICU admission [12].
Fasting plasma glucose as screening test
In our study the sensitivity and specificity of FPG at various cutoffs was drawn on ROC curve and a cutoff level of 84.5 mg/dL had the highest sensitivity (93.8%), specificity (74.3%), The positive predictive and negative predictive values were 20.3% and 99.4% respectively. In comparison Reichelt AJ et al., found that a cut point of 85 mg/dL maximizes sensitivity (94%) without undue loss of specificity (66%) [21]. In comparison Percchini D et al., observed that using receiver operating curves best cutoff value for using FPG concentration as a screening test for gestational diabetes was 4.8 mmol/L (86.4 mg/dL) as it yielded a sensitivity of 81% and a specificity of 76% [22].
Limitation
The study was conducted in a single centre which may not be a representative of whole population as we had selected only 246 patients, this might influence the results. Also patients were generally from low socioeconomic group with poor nutrition so the high risk factors responsible for GDM such as obesity was not observed which might affect the results.
Conclusion
As the prevalence of GDM is increasing in India, identifying the women with GDM is important to avoid maternal and neonatal complications. FPG on first antenatal visit is useful as a screening test to diagnose GDM as it is a simple, easily available, reproducible and cost-effective test in a system like ours where health resource is scarce. Early diagnosis of the disease and early intervention will always improve the pregnancy outcome.
1Chi-square test, *Significant, GDM-Gestational Diabetes Mellitus, NGT-Normal Glucose Tolerance
1Unpaired t-test, *Significant, FPG-Fasting Plasma Glucose GDM-Gestational Diabetes Mellitus, NGT-Normal Glucose Tolerance
1Chi-square test, *Significant, GDM-Gestational Diabetes Mellitus, NGT-Normal Glucose Tolerance
1Pearson correlation analysis, *Significant, GDM-Gestational Diabetes Mellitus, OGTT-Oral Glucose Tolerance Test, NGT-Normal Glucose Tolerance
LR- Likelihood ratio, AUC- Area under the curve. The AUC (95% CI) was 0.96 (0.92-0.99) with p=0.0001, PPV-Positive Predictive Value, NPV-Negative Predictive Value
FPG-Fasting Plasma Glucose, GDM-Gestational Diabetes Mellitus