Primary Early Surgical Management of Pancreatic Ascites Complicating Chronic Pancreatitis-A Single Centre Experience
Ramalingam Durai Rajan Somasekar1, Raju Prabhakaran2, Anbalagan Amudhan3, Murugaiyan Gnanasekar4, Kalyanashanmugam Sivakumar5, Govindaraj Raman Senthilkumaran6, Shanmugasundaram Rajendran7, Obla Naganathbabu8
1 Postgraduate, Institute of Surgical Gastroenterology, Madras Medical College, Chennai, Tamil Nadu, India.
2 Assistant Professor, Institute of Surgical Gastroenterology, Madras Medical College, Chennai, Tamil Nadu, India.
3 Assistant Professor, Institute of Surgical Gastroenterology, Madras Medical College, Chennai, Tamil Nadu, India.
4 Assistant Professor, Institute of Surgical Gastroenterology, Madras Medical College, Chennai, Tamil Nadu, India.
5 Assistant Professor, Institute of Surgical Gastroenterology, Madras Medical College, Chennai, Tamil Nadu, India.
6 Postgraduate, Institute of Surgical Gastroenterology, Madras Medical College, Chennai, Tamil Nadu, India.
7 Professor, Institute of Surgical Gastroenterology, Chennai, Tamil Nadu, India.
8 Director and Professor, Institute of Surgical Gastroenterology, Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Obla Naganathbabu, Room No. 244A, Tower II, 4th Floor, Institute of Surgical Gastroenterology, Rajiv Gandhi Government General Hospital, Chennai-600003, Tamil Nadu, India.
E-mail: naganathbabu@gmail.com
Introduction
The traditional method of managing Pancreatic Ascites (PA) complicating Chronic Pancreatitis (CP) was with initial conservative treatment which was associated with increased morbidity and mortality.
Aim
To describe about the new treatment protocol which lays emphasis on primary early surgical intervention for PA complicating advanced CP cases based on the pathological morphology of the disease instead of an initial trial of conservative treatment.
Materials and Methods
This was a prospective observational study of 15 cases of CP with PA managed over a three year period. The approach was guided by the pathological morphology defined by a CECT abdomen and/or Magnetic Resonance Cholangiopancreatography (MRCP). Of the observed 15 cases, imaging showed a dilated Main Pancreatic Duct (MPD) 5-10 mm in 11 cases, ductal disruption in 4/11 cases and pseudocyst in 8/11 cases. These 11 cases underwent primary early direct surgery. Surgery was tailored to the individual case with a combination of internal ductal/pseudocyst drainage and/or distal resection. Resolution of PA and relief of symptoms were the primary outcome measures. Recurrence of PA at one year follow up after surgery was the secondary outcome measure.
Results
Resolution of PA and relief of symptoms occurred in all patients in the primary surgery group. The mean duration of hospital stay was 16 days in the primary surgery group with a range of nine to 23 days with no mortality and no disease recurrence after one year of follow up.
Conclusion
Primary early direct surgery guided by the MPD morphology (duct diameter >5 mm) in selected patients with CP and PA leads to faster recovery of the patient and it takes care of the primary pathology too.
Fistula, Internal pancreatic fistula, Pancreatic duct disruption, Pseudocyst
Introduction
Pancreatic Ascites was first reported in the literature by Smith in 1953 [1]. It is defined as an exudative ascites caused by non-malignant pancreatic disease and is characterised by a very high amylase concentration in ascitic fluid (usually over 1000 IU/L) and albumin concentration over 3 gm/dL [2]. PA can occur as a complication of either acute or CP. In either case, it can be as a result of a leaking pseudocyst communicating with the MPD or as a result of anterior disruption of the MPD. PA is an infrequent complication and the exact incidence is not known. It is seen in 3.5% of patients with chronic pancreatitis and 6-14% of patients with pseudocysts [3]. In Broe and Cameroon series it occurred most frequently in patients with CP and in most cases it was associated with a pseudocyst. The pancreatic enzymes in the ascitic fluid are inactive and do not lead to digestion of tissues, but instead causes inflammation and exudation leading to an albumin rich fluid. In patients with severe hypoproteinaemia, the ascitic fluid albumin levels might be less than 3 gm/dL [2].
There are three approaches in treating these patients namely a) initial trial of conservative therapy with repeated paracentesis, Total Parenteral Nutrition (TPN) and octreotide use b) endotherapy and c) surgery [3]. On going through the available literature there is consistently a trend towards initial trial of conservative therapy with endotherapy and surgery reserved in case of failure of conservative management. There are no randomised trials comparing the efficacy of each modality and this might possibly be due to the limited number of available cases. In CP, an initial trial of conservative therapy is unlikely to be fruitful as the MPD is already diseased and obstructed. Endotherapy for ductal disruptions in CP has its own limitations in terms of availability of expertise and feasibility. Endotherapy may not be successful in patients with multiple ductal strictures or intraductal calculi. There appears to be considerable nihilism in resorting directly to surgery in patients with PA complicating CP as these patients are nutritionally depleted and hence, consequent fear of increased morbidity and mortality. We here report a study with 11 cases of CP with PA treated with primary direct surgery.
Materials and Methods
This was a prospective observational study conducted between 1st January 2012 to 31st December 2015 at the Institute of Surgical Gastroenterology, Madras Medical College and approval from the Institutional Ethical Committee had been obtained. An informed consent had been obtained from all the patients participating in the study. The inclusion criterion was that all patients were with chronic pancreatitis with ascites and an ascitic fluid amylase level >1000 IU/L. We have treated a total of 22 cases of pancreatic ascites over a three year period from 2012 to 2015 of which 15 cases (68%) were due to CP, five cases (23%) were following acute necrotising pancreatitis and two cases (9%) following blunt abdominal trauma. In 14 (93%) of 15 cases the aetiology was ethanol induced CP and all were males. One female patient had idiopathic CP. The age of the patients ranged between 16 to 48 years. Of the 15 cases with CP and PA, a previous episode of pancreatitis was seen in eight (53%) patients. Seven (47%)/15 cases had no previous episodes of abdominal pain and were presenting with painless abdominal distension between one to three months duration with or without weight loss [Table/Fig-1].
| Symptoms | No. of patients out of 15 CP cases |
|---|
| Abdomen pain+distension | 7 |
| Abdomen pain alone | 1 |
| Abdominal distention alone | 3 |
| Abdominal distension and weight loss | 4 |
| Loss of appetite | 12 |
| Steatorrhoea | 3 |
| Previous episodes of pancreatitis | 8 |
| Diabetes | 4 |
CP-Chronic pancreatitis
All the 15 patients were hospitalised and evaluated with the following investigations: Complete Blood Count (CBC), Renal Function Test (RFT), Liver Function Tests (LFT), serum amylase, ascitic fluid amylase and albumin levels, plain X-ray chest to rule out coexistent pleural effusion, electrocardiogram, ultrasound of the abdomen, a pancreatic protocol Multidectector Computed Tomography (MDCT) of the abdomen and an MRI abdomen with MRCP. All patients were subjected to cardiac evaluation with an echocardiography and a Pulmonary Function Test (PFT). All patients were encouraged to do incentive spirometry to improve the pulmonary compliance. Patients with a dilated MPD more than or equal to 5 mm (11/15 patients) and with good cardiopulmonary reserve were selected for primary surgery. The rest of the four patients without a dilated MPD were subjected to an ultrasound guided percutaneous catheter insertion for continuous drainage of the ascitic fluid and were further subjected to imaging to delineate the site of MPD disruption. Two of these four patients were able to tolerate oral feeds after percutaneous catheter drainage, which improved their comfort level and were put on enteral hyperalimentation. In the other two of the four patients without MPD dilatation who were not able to tolerate oral feeds, a nasojejunal tube was inserted for enteral hyperalimentation. Among the 11 patients with MPD dilatation, selective use of paracentesis was done when the patient developed respiratory distress. Surgery was tailored to the individual case with a combination of internal duct/pseudocyst drainage and/or distal resection. The patient profiles of the 15 cases are as shown in [Table/Fig-2].
Patient profiles of the 15 cases.
| S. No. | Age (years)/sex | Clinical presentation | Serum amylase IU/L | Ascitic fluid amylase IU/L | Ascitic fluid albumin gm/dL |
|---|
| 1. | 20/M | Abdominal pain and distension | 340 | 2052 | 3.1 |
| 2. | 40/M | Abdominal pain and distension | 412 | 5662 | 2.8 |
| 3. | 21/M | Abdominal pain and distension | 257 | 3067 | 2.9 |
| 4. | 43/M | Abdominal pain and distension | 670 | 14000 | 3.5 |
| 5. | 48/M | Abdominal distension/weight loss | 350 | 5780 | 3.2 |
| 6. | 16/F | Abdominal distension/weight loss | 650 | 2430 | 3.1 |
| 7. | 46/M | Abdominal distention/weight loss | 430 | 18000 | 3.6 |
| 8 | 37/M | Abdominal distension alone | 317 | 7440 | 3.2 |
| 9. | 24/M | Abdomen pain, distension, weight loss | 420 | 3450 | 3.5 |
| 10. | 40/M | Abdomen distension alone | 560 | 6400 | 3.1 |
| 11. | 36/M | Abdomen distension alone | 250 | 4000 | 3.2 |
| 12. | 36/M | Abdominal pain and distension | 439 | 3730 | 2.5 |
| 13. | 33/M | Abdominal pain and distension | 1021 | 4749 | 2.3 |
| 14. | 40/M | Abdominal pain alone | 499 | 3692 | 3.8 |
| 15. | 36/M | Abdominal pain and distension | 400 | 6700 | 3.1 |
Results
Pancreatic protocol MDCT abdomen and MRCP showed dilated MPD in 11 of 15 cases. The duct diameter ranged from 5-10 mm on imaging. There was associated pseudocyst in 8/11 cases. The site of MPD disruption was identified in 4/11 cases, all of which were in the distal pancreas to the left of the superior mesenteric artery. The 11/15 cases underwent primary surgery as follows:
Lateral Pancreaticojejunostomy (LPJ)-6
Cystogastrostomy-2
Distal pancreatectomy with splenectomy and LPJ to the remnant-3.
The abdomen was opened by a roof top incision and all the ascitic fluid suctioned out. In 9/11 cases there were flimsy adhesions between the small bowel loops. In all the patients entry into the lesser sac was done by opening the gastrocolic omentum and in four there were dense adhesions between the posterior wall of stomach and pancreas, which were released. In the three patients who underwent distal resection of the pancreas, the pancreatic parenchyma of the body and tail was not conducive for a safe anastomosis in one patient and there was a coexistent pseudocyst in the tail of the pancreas in two patients. In these three patients the spleen was removed along with the pancreas due to dense adhesions in two and inadvertent injury in one patient.
In another 2/11 cases a pseudocyst communicating with a dilated MPD was identified but the site of pseudocyst leakage or MPD disruption could not be made out. These two patients underwent a cystogastrostomy. In 6/11 cases that underwent a LPJ, MPD disruption was identified in only two cases and four patients had an associated small pseudocyst communicating with the dilated MPD. These communicating pseudocysts were small and laid open to include in the anastomosis.
In the post operative period, four patients had Grade A pancreatic fistula and settled with conservative management. Four patients had pulmonary atelectasis and managed with non invasive mechanical ventilation. Three had surgical site infection and settled with appropriate antibiotics. All the 11/15 patients in the primary surgery group had some form of morbidity that was acceptable and manageable because of the poor nutritional status. There was no mortality. Pneumococcal vaccine was given preoperatively in 2/3 patients who had a pseudocyst in the tail of the pancreas at the splenic hilum and underwent a distal resection with splenectomy. In the other splenectomised patient the vaccine was given postoperatively. The duration of hospital stay ranged from nine to 23 days since admission in the above 11/15 patients who underwent primary surgery. A snapshot of the pathological morphology of the disease and the surgery done is given in [Table/Fig-3].
Pathological morphology of the disease and the surgery done.
| S. No. | No. and location of pseudocyst (size in cm) | Site of MPD disruption/leaking pseudocyst on imaging/MPD calculi | Site of disruption on MDCT identified | Site of disruption on MRCP identified | MPD Diameter | Procedure done |
|---|
| 1. | One/tail of pancreas (6 cm) | Leaking pseudocyst/intraductal caculi+ | Yes | Yes | 7 mm | Distal pancreatectomy+splenectomy and LPJ to the remnant |
| 2. | Nil | MPD disruption in distal body/intraductal calculi+ | Yes | Yes | 8 mm | Distal pancreatectomy+Splenectomy and LPJ to the remnant |
| 3. | One/tail of pancreas (10 cm) | Leaking pseudocyst communicating with MPD disruption in the tail/intraductal calculi+ | No | Yes | 10 mm | Distal pancreatectomy+splenectomy and LPJ to the remnant |
| 4. | One/head of pancreas (2 cm) | Could not be identified/intraductal calculi+ | No | No | 9 mm | LPJ |
| 5. | One/head of pancreas (3 cm) | Could not be identified/intraductal calculi + | No | No | 8 mm | LPJ |
| 6. | One/tail of pancreas (2 cm) | Could not be identified/intraductal calculi+ | No | No | 10 mm | LPJ |
| 7. | Nil | MPD disruption in body of pancreas/intraductal calculi+ | Yes | Yes | 7 mm | LPJ |
| 8. | One/body of pancreas (2 cm) | MPD disruption in body of pancreas/intraductal calculi+ | No | Yes | 9 mm | LPJ |
| 9. | Nil | Could not be identified/intraductal calculi+ | No | No | 8 mm | LPJ |
| 10. | One/body of pancreas (7 cm) | Could not be identified/no ductal calculi/specs of parenchymal calcification+ | No | No | 5 mm | Cystogastrostomy |
| 11. | One/body of pancreas (8 cm) | Could not be identified/no intraductal calculi/specs of parenchymal calcification+ | No | No | 5 mm | Cystogastrostomy |
| 12. | One/head of pancreas (2 cm) | Could not be identified/no intraductal calculi/specs of parenchymal calcification+ | No | No | Not dilated | Conservative management |
| 13. | Nil | MPD disruption near distal body close to tail/no intraductal calculi/specs of parenchymal calcification+ | Yes | Yes | Not dilated | Resolved with initial conservative therapy. Had recurrent PA managed with MPD stenting and failed. Later underwent distal pancreatectomy+splenectomy |
| 14. | One/head of pancreas (2 cm) | Leaking pseudocyst/no intraductal calculi/parenchymal calcification+ | No | Yes | Not dilated | Conservative management |
| 15. | One/tail of pancreas (3 cm) | Could not be identified/no intraductal calculi/parenchymal calcification+ | No | No | Not dilated | Conservative management |
MPD-Main pancreatic duct, MDCT-Multidectector computed tomography, MRCP-Magnetic resonance cholangiopancreatography, PA-Pancreatic ascites, LPJ-Lateral pancreaticojejunostomy
The other 4/15, which had CP+PA without MPD dilatation [Table/Fig-4,5,6 and 7] were managed with percutaneous catheter drainage and early enteral feeds. The duration of hospital stay ranged from 24-35 days.
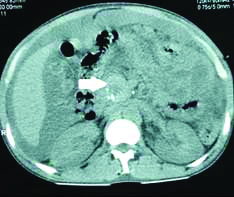
Plain CT abdomen showing ascites with specs of parechymal caicificition and a cystic area in the uncinate process of the panceas (arrow).
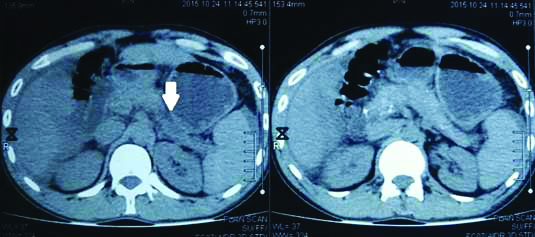
Plain CT abdomen showing in the site of parechymal disruption in the body (arrow) in the left hand side picture and specs of parechyma calcification in the right hand side picture.
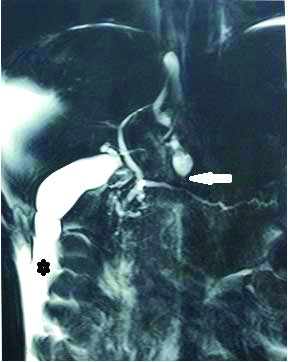
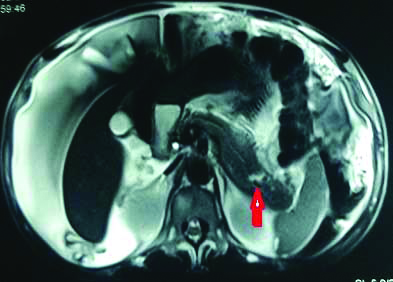
T2 weighted MRCP image showing MPD disruption in body with leak of pancreatic fluid (arrow) into the penitoneal cavity with ascites (star). MRCP-Magnetic resonance cholangiopancreatography, MPD-Main pancreatic duct
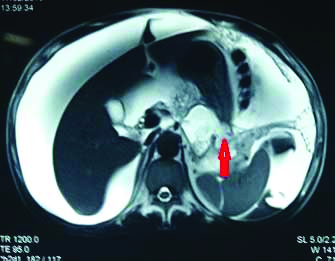
T2 weighted MRI, an axial cut showing a pseudocyst in the body of pancreas with rupture (arrow) with fluid tracking intopenitoneal cavity.
At six months and one year follow up there was no recurrence of ascites in all the 11/15 patients who underwent primary surgery and all were free of symptoms. Among the 4/15 patients managed conservatively one had recurrent ascites managed with endotherapy initially but failed and later underwent distal pancreatectomy with splenectomy for distal ductal disruption [Table/Fig-8]. Of the other three of the four patients there was no recurrence of ascites but two had recurrent hospital admission for acute attack on CP. These two patients were not compliant with alcohol abstinence and were subjected to de-addiction therapy.
T2 weighted MRI, an axial cut showing site of MPD disruption (arrow) with tracking of fluid into penitoneal cavity. MPD-Main pancreatic duct.
Discussion
Internal pancreatic fistulas are a rare complication of pancreatitis, either acute or chronic. In our series, it is more common in males and in the setting of ethanol induced CP (15/22 cases 68%) rather than acute pancreatitis. Our observation is in concordance with the John Hopkins University experience, one of the largest reported series till date [4]. A logical explanation for this is that in acute pancreatitis the surrounding inflammation walls off the site of disruption to form a localised collection which later matures to form a pseudocyst or resolves. The MPD is not obstructed or diseased in acute pancreatitis. In CP the parenchyma is atrophic and fibrous, the MPD is obstructed leading to increased intraductal pressure and disruption.
With regard to clinical presentation, 7/15 cases (47%) had neither abdominal pain nor a previous documented episode of pancreatitis. These seven cases presented with gradually progressive painless abdominal distension with or without weight loss. On clinical examination, these seven patients had no abdominal tenderness. five of these seven patients were previously misdiagnosed as ethanol induced chronic liver disease elsewhere. In the other 8/15 patients mild abdominal tenderness was present, but the clinical examination was otherwise unproductive. In our series, nearly half of the patients had no prior episodes of pancreatitis posing a diagnostic dilemma. A high index of suspicion even in the absence of a prior history of pancreatitis and ascitic fluid analysis for amylase and albumin levels will help to clinch the diagnosis. As majority of these patients have chronic history of alcohol consumption, there can possibly be coexistent chronic liver disease.
In 40-80% of cases the PA is due to a leaking pseudocyst communicating with the main pancreatic duct, in 10% due to MPD disruptions without a pseudocyst and in another 10% the site of disruption cannot be identified [5]. In our series, there was a communicating pseudocyst in 11/15 (73%) cases [Table/Fig-9]. All the identified MPD disruptions were distal. This is possibly because of the atrophic and thinned out parenchyma in the body and tail region making them weaker spots for disruption as compared to the head region where the volume of parenchyma is relatively more. In PA identifying the site of MPD disruption helps to plan appropriate therapy especially when the MPD is not dilated. In advanced CP+PA where the MPD is sufficiently dilated (>or=5 mm) to do a safe internal drainage procedure, identifying the site of disruption is just an adjunct to plan therapy and not mandatory [6]. In our series, MDCT abdomen was instrumental in identifying the site of disruption in 4/15 (27%) cases [Table/Fig-10]. When an MRCP was added to the diagnostic armamentarium, the site of disruption was localised in three more cases. A secretin enhanced MRCP better delineates the ductal anatomy [7]. A disruption could not be identified with both imaging in 8/15 patients. A pancreatic protocol MDCT and an MRI abdomen with MRCP are complementary to each other in localising the site of duct/pseudocyst disruption [8]. An Endoscopic Retrograde Cholngiopancreatography (ERCP) may be contemplated both as a diagnostic and therapeutic procedure when both imaging modalities fail to identify the site of ductal disruption and the MPD is not sufficiently dilated to do a safe duct drainage procedure.
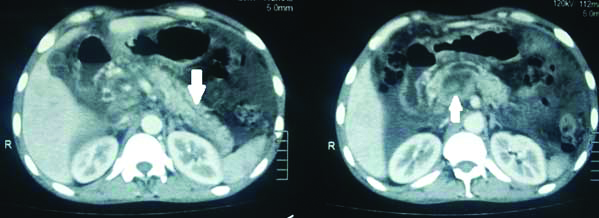
Contrast enhanced MDCT abdomen showing dilated MPD (arrow) in the body of pancreas on the left picture and dilated MPD in the head of pancreas with intraparenchymal pseudocyst in the head (arrow) on the right picture. MDCT-Multidectector computed tomography, MPD-Main pancreatic duct
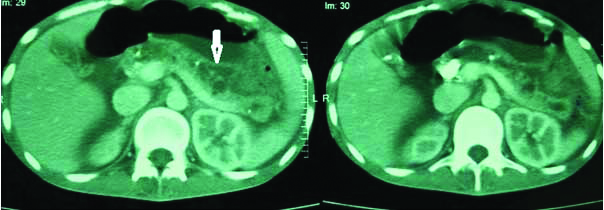
Contrast enhanced MDCT abdomen showing dilated MPD with disruption in the body (arrow) on the left picture and a large chunky calculus in the head on the right picture. MDCT-Multidectector computed tomography, MPD-Main pancreatic duct
Conservative therapy for pancreatitis consists in keeping the patient nil per oral and use of somatostatin analogues [9,10] to decrease the pancreatic secretions. Repeated large volume paracentesis is done to improve patient discomfort and also with a premise that this might promote approximation of peritoneal surfaces of the lesser sac to the leaking site thereby, sealing the site of ductal or pseudocyst disruption. In our study, we adopted a strategy of percutaneous catheter drainage of the ascitic fluid and early enteral hyperalimentation either orally or using nasojejunal tube in 4/15 cases without MPD dilatation. We also selectively used 100 mL of 20% human albumin infusion per day for three consecutive days as part of our conservative treatment protocol when the serum albumin levels were below 3 gm/dL. This cut-off is an arbitrary one and is based on our preference. The rationale behind this is albumin infusions help to increase the colloid osmotic pressure of the intravascular compartment as majority of these patients are hypoproteinaemic. It is our speculation that this either prevents further fluid shifts into the peritoneum or promotes reabsorption of the fluid accumulated in the peritoneal cavity. We have found this very effective and probably will be precedence for other investigators to use this protocol. We have found this protocol very effective in patients with PA complicating acute pancreatitis as well.
Endotherapy with pancreatic duct stenting is a viable option in patients with PA complicating CP and also for post traumatic MPD disruptions [11]. MPD stenting for ductal disruptions has pros and cons. The various reported series [12-21] claim success rates from 80-100%. However, this is limited by availability of expertise, feasibility of stenting the disruption and also the location of the ductal disruption. Moreover, pancreatic duct stenting has its own risk of iatrogenic complications like side-branch occlusion, parenchymal atrophy and glandular fibrosis [22,23]. The side flap of the stent can induce ductitis and cause duct stenosis as a result of fibrosis. Above all MPD stenting leads to bacterial colonisation and infectious complications [24]. This will make future surgery difficult in cases where endotherapy fails. In patients with advanced CP with multiple duct strictures and intraductal calculi the role of endotherapy is limited . When the MPD is dilated sufficiently to do a safe internal ductal drainage procedure, surgery addresses both the ductal disruption and the associated ductal pathology (strictures and calculi). In patients with intractable pain due to CP and PA surgery again addresses both.
There are various surgical options that must be tailored to the individual case depending on the pathological morphology of the disease and fitness of the patient for surgery. In majority of the cases there is an associated communicating pseudocyst which is usually small, possibly due to the constant leakage of pancreatic juice into the peritoneal cavity. When there is a communicating pseudocyst in the tail a distal pancreatic resection or internal drainage of the pseudocyst can be done [Table/Fig-11]. The proximal remnant pancreatic duct is drained internally when diseased and obstructed [Table/Fig-12]. When there is distal ductal disruption alone, a distal resection will suffice [Table/Fig-13,14]. The extent of parenchyma resection should always be kept to a minimum to delay the onset of endocrine or exocrine insufficiency in an already diseased organ. When there is a diffuse MPD dilation with disruption, an LPJ is the most appropriate procedure irrespective of the site of disruption. The cavity of a small pseudocyst communicating with the MPD can be included in the LPJ. In case of proximal ductal disruptions without a dilated MPD a Roux-en Y fistulojejunostomy can be done. When a pseudocyst alone is present an internal drainage procedure to the stomach, duodenum or Roux loop of jejunum is appropriate. If the pseudocyst wall is thin and not safe for an anastomosis an external drainage procedure can be done. The reported success rate with the various series is >90% [25-29]. The overall mortality rate is 9% [30]. In our series, we had no mortality and all the 11/15 patients had no disease recurrence at the end of one year of post-operative follow up. The duration of hospital stay ranged from nine to 23 days. A pragmatic algorithm for management of PA is given below [Table/Fig-15].
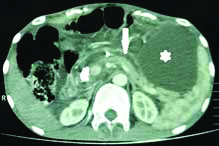
Contrast enhanced MDCT abdomen showing dilated MPD (vertical arrow) with inraductal calculi and a pseudocyst in the tail (star). MDCT-Multidectector computed tomography, MPD-Main pancreatic duct
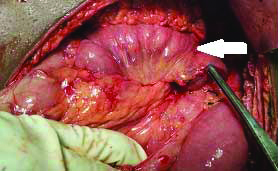
Intraoperative picture showing the pancreaticojejunal anastomosis to the remnant head of pancreas after distal pancreatectomy+splenectomy
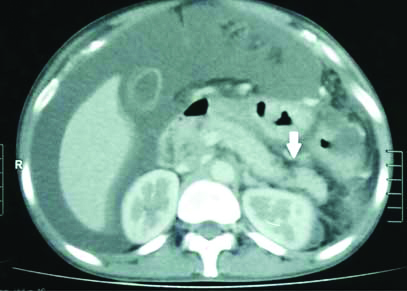
Contrast enhanced MDCT abdomen showing prominent MPD with distal disruption (white arrow) with massive ascites. MDCT-Multidectector computed tomography, MPD-Main pancreatic duct
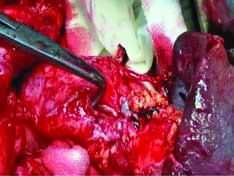
Intraoperative photo showing MPD disruption in distal pancreas close to the tail with the spleen in the left corner of the picture. MPD-Main pancreatic duct
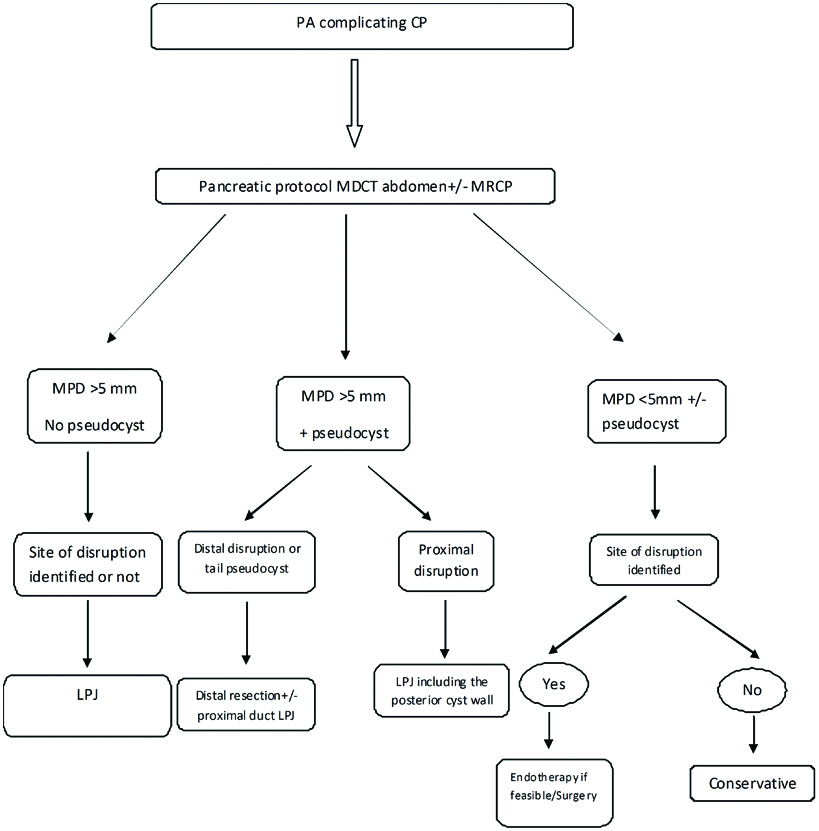
A pragmatic algorithm for management of pancreatic ascites (PA) in chronic pancreatitis (CP). MDCT- Multidectector computed tomography, MRCP- Magnetic resonance cholangiopancreatography, MPD-Main pancreatic duct
Limitation
The above study is a prospective observational study design. Future randomised controlled trials comparing primary early direct surgery to either conservative therapy or endotherapy are warranted to test the strength of our observations. However, this is limited by the difficulty in accrual of patients because of the rarity of PA.
Conclusion
A high-index of clinical suspicion especially in chronic ethanol users with painless abdominal distension with analysis of ascitic fluid amylase and albumin helps to pin point the diagnosis. A pancreatic protocol MDCT abdomen and an MRCP are complementary to each other in localising the site of MPD disruption. In patients with a dilated MPD sufficient to do a safe internal drainage procedure, localising the site of ductal disruption is not mandatory. The novel method of selective use of albumin infusion as part of the conservative treatment protocol yields good results. Primary early direct surgery guided by the MPD morphology in selected patients with CP and PA leads to faster recovery of the patient and it takes care of the primary pathology too.
CP-Chronic pancreatitis
MPD-Main pancreatic duct, MDCT-Multidectector computed tomography, MRCP-Magnetic resonance cholangiopancreatography, PA-Pancreatic ascites, LPJ-Lateral pancreaticojejunostomy
[1]. Smith E, Hemorrhagic ascites and hemothorax associated with benign pancreatic diseaseAMA Archives of Surgery 1953 67(1):52-56.10.1001/archsurg.1953.0126004005500813064942 [Google Scholar] [CrossRef] [PubMed]
[2]. Cameron J, Kieffer R, Anderson W, Zuidema G, Internal Pancreatic FistulasAnnals of Surgery 1976 184(5):587-93.10.1097/00000658-197611000-00009984927 [Google Scholar] [CrossRef] [PubMed]
[3]. Gómez-Cerezo J, Barbado Cano A, Suárez I, Soto A, Ríos JJ, Vázquez JJ, Pancreatic ascites: Study of therapeutic options by analysis of case reports and case series between the years 1975 and 2000The Am J Gastroenterol 2003 98(3):568-77.10.1016/S0002-9270(02)06032-X [Google Scholar] [CrossRef]
[4]. Lipsett P, Cameron J, Internal pancreatic fistulaThe Am J Surg 1992 163(2):216-20.10.1016/0002-9610(92)90104-Y [Google Scholar] [CrossRef]
[5]. Kurumboor P, Varma D, Rajan M, Kamlesh N, Paulose R, Narayanan R, Outcome of pancreatic ascites in patients with tropical calcific pancreatitis managed using a uniform treatment protocolIndian J Gastroenterol 2009 28(3):102-06.10.1007/s12664-009-0037-919907961 [Google Scholar] [CrossRef] [PubMed]
[6]. Cahen D, Gouma D, Nio Y, Rauws E, Boermeester M, Busch O, Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitisN Eng J Med 2007 356(7):676-84.10.1056/NEJMoa06061017301298 [Google Scholar] [CrossRef] [PubMed]
[7]. Gillams A, Kurzawinski T, Lees W, Diagnosis of duct disruption and assessment of pancreatic leak with dynamic secretin-stimulated MR cholangiopancreatographyAm J Roentgenol 2006 186(2):499-506.10.2214/AJR.04.177516423959 [Google Scholar] [CrossRef] [PubMed]
[8]. O’Toole D, Vullierme M, Ponsot P, Maire F, Calmels V, Hentic O, Diagnosis and management of pancreatic fistulae resulting in pancreatic ascites or pleural effusions in the era of helical CT and magnetic resonance imagingGastroentérologie Clinique et Biologique 2007 31(8-9):686-93.10.1016/S0399-8320(07)91918-1 [Google Scholar] [CrossRef]
[9]. Parekh D, Pancreatic ascites and effusionArchives of surgery 1992 127(6):70710.1001/archsurg.1992.014200600830121596172 [Google Scholar] [CrossRef] [PubMed]
[10]. Segal I, Parekh D, Lipschitz J, Gecelter G, Myburgh J, Treatment of pancreatic ascites and external pancreatic fistulas with a long-acting somatostatin analogue (Sandostatin)Digestion 1993 54(1):53-58.10.1159/0002010788359569 [Google Scholar] [CrossRef] [PubMed]
[11]. Subramanian A, Dente C, Feliciano D, The management of pancreatic trauma in the modern eraSurgical Clinics of North America 2007 87(6):1515-32.10.1016/j.suc.2007.08.00718053845 [Google Scholar] [CrossRef] [PubMed]
[12]. Bhasin D, Rana S, Siyad I, Poddar U, Thapa B, Sinha S, Endoscopic transpapillary nasopancreatic drainage alone to treat pancreatic ascites and pleural effusionJ Gastroenterol and Hepatol 2006 21(6):1059-64.10.1111/j.1440-1746.2005.04049.x16724995 [Google Scholar] [CrossRef] [PubMed]
[13]. Bracher G, Manocha A, DeBanto J, Gates L, Slivka A, Whitcomb D, Endoscopic pancreatic duct stenting to treat pancreatic ascitesGastrointestinal Endoscopy 1999 49(6):710-15.10.1016/S0016-5107(99)70287-7 [Google Scholar] [CrossRef]
[14]. Chebli J, Gaburri P, Meirelles de Souza A, Ornellas A, Junior E, Chebli L, Internal pancreatic fistulasJ Clin Gastroenterol 2004 38(9):795-800.10.1097/01.mcg.0000139051.74801.4315365408 [Google Scholar] [CrossRef] [PubMed]
[15]. Kozarek R, Jiranek G, William Traverso L, Endoscopic treatment of pancreatic ascitesThe Am J Surg 1994 168(3):223-26.10.1016/S0002-9610(05)80190-4 [Google Scholar] [CrossRef]
[16]. Pai C, Suvarna D, Bhat G, Endoscopic treatment as first-line therapy for pancreatic ascites and pleural effusionJ Gastroenterol and Hepatol 2009 24(7):1198-1202.10.1111/j.1440-1746.2009.05796.x19486258 [Google Scholar] [CrossRef] [PubMed]
[17]. Cicek B, Parlak E, Oguz D, Disibeyaz S, Koksal A, Sahin B, Endoscopic treatment of pancreatic fistulasSurgical Endoscopy 2006 20(11):1706-12.10.1007/s00464-005-0764-716960673 [Google Scholar] [CrossRef] [PubMed]
[18]. Miyachi A, Kikuyama M, Matsubayashi Y, Kageyama F, Sumiyoshi S, Kobayashi Y, Successful treatment of pancreaticopleural fistula by nasopancreatic drainage and endoscopic removal of pancreatic duct calculi: a case reportGastrointestinal Endoscopy 2004 59(3):454-57.10.1016/S0016-5107(03)02719-6 [Google Scholar] [CrossRef]
[19]. Neher J, Brady P, Pinkas H, Ramos M, Pancreaticopleural fistula in chronic pancreatitis: resolution with endoscopic therapyGastrointestinal Endoscopy 2000 52(3):416-18.10.1067/mge.2000.10829610968864 [Google Scholar] [CrossRef] [PubMed]
[20]. Halttunen J, Weckman L, Kemppainen E, Kylänpää M, The endoscopic management of pancreatic fistulasSurgical Endoscopy 2005 19(4):559-62.10.1007/s00464-004-9140-215696357 [Google Scholar] [CrossRef] [PubMed]
[21]. Varadarajulu S, Noone T, Tutuian R, Hawes R, Cotton P, Predictors of outcome in pancreatic duct disruption managed by endoscopic transpapillary stent placementGastrointestinal Endoscopy 2005 61(4):568-75.10.1016/S0016-5107(04)02832-9 [Google Scholar] [CrossRef]
[22]. Smith M, Sherman S, Ikenberry S, Hawes R, Lehman G, Alterations in pancreatic ductal morphology following polyethylene pancreatic stent therapyGastrointestinal Endoscopy 1996 44(3):268-75.10.1016/S0016-5107(96)70163-3 [Google Scholar] [CrossRef]
[23]. Kozarek R, Pancreatic stents can induce ductal changes consistent with chronic pancreatitisGastrointestinal Endoscopy 1990 36(2):93-95.10.1016/S0016-5107(90)70958-3 [Google Scholar] [CrossRef]
[24]. Kozarek R, Hovde O, Attia F, France R, Do pancreatic duct stents cause or prevent pancreatic sepsis?Gastrointestinal Endoscopy 2003 58(4):505-09.10.1067/S0016-5107(03)01891-1 [Google Scholar] [CrossRef]
[25]. Martin F, Rossi R, Munson JL, Management of pancreatic fistulasArchives of Surgery 1989 124(5):571-73.10.1001/archsurg.1989.014100500610122712699 [Google Scholar] [CrossRef] [PubMed]
[26]. Pottmeyer E, Frey CF, Matsuno S, Pancreaticopleural fistulasArchives of Surgery 1987 122(6):648-54.10.1001/archsurg.1987.014001800300063579578 [Google Scholar] [CrossRef] [PubMed]
[27]. Varadarajulu S, Rana S, Bhasin D, Endoscopic Therapy for Pancreatic Duct Leaks and DisruptionsGastrointestinal Endoscopy Clinics of North America 2013 23(4):863-92.10.1016/j.giec.2013.06.00824079795 [Google Scholar] [CrossRef] [PubMed]
[28]. Dhar P, Tomey S, Jain P, Azfar M, Sachdev A, Chaudhary A, Internal pancreatic fistulae with serous effusions in chronic pancreatitisANZ Journal of Surgery 1996 66(9):608-11.10.1111/j.1445-2197.1996.tb00830.x8859161 [Google Scholar] [CrossRef] [PubMed]
[29]. Eckhauser F, Raper S, Knol J, Mulholland M, Surgical management of pancreatic pseudocysts, pancreatic ascites, and pancreaticopleural fistulasPancreas 1991 6:S66-75.10.1097/00006676-199101001-000111788255 [Google Scholar] [CrossRef] [PubMed]
[30]. Alexakis N, Sutton R, Neoptolemos J, Surgical treatment of pancreatic fistulaDigestive Surgery 2004 21(4):262-74.10.1159/00008019915308865 [Google Scholar] [CrossRef] [PubMed]