Asthma is the commonest chronic disorder in children associated with significant activity limitation and accounts for estimated 14.4 million lost school days in children [1]. WHO has estimated that 10% to 15% of Indian children in the age group 5-11 years are asthmatics [2].
Corticosteroids play an important role in the treatment of acute exacerbation of asthma. Several guidelines including British Thoracic Society (BTS) and Global Initiative for Asthma (GINA) recommend oral prednisolone as early as possible to children presenting with acute asthma exacerbation [3,4]. Recently, oral dexamethasone has been evaluated as an alternative for prednisolone and studies have found that it is as effective as prednisolone in the treatment of acute asthma exacerbations [5-7]. Dexamethasone’s anti-inflammatory effect is six times more potent than prednisolone and its half-life (36-72 hours) is at least twice as long as that of the prednisolone (12-36 hours); also, its absorption from the gastrointestinal tract and its bioavailability are good [8]. Thus, theoretically, even a single dose of dexamethasone would be enough in place of multiple doses of prednisolone. Though, there are enough studies to support the equivalent efficacy of single dose of dexamethasone to three day course of prednisolone, there is paucity of such studies in India. Also, the existing guidelines do not seem to support dexamethasone over prednisolone. Hence, we decided to study the same in our setting.
Materials and Methods
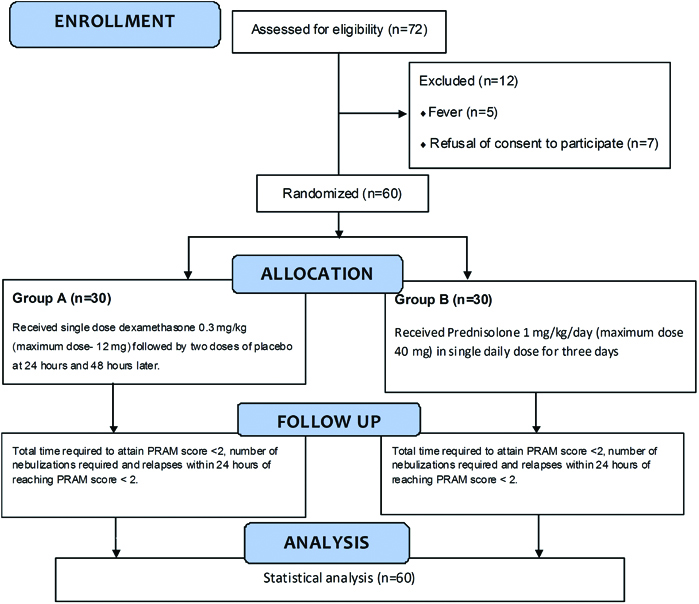
This double blinded (the principal investigator who followed up the child till discharge and the study subjects were blinded to the intervention being received), randomised controlled trial study with a placebo control was conducted at a tertiary care medical college and hospital in Southern India over a period of 10 months from November 2015 to August 2016. After getting the Institute ethics committee approval, a convenient sample of 60 bronchial asthma children aged two to twelve years, presenting with acute exacerbation of moderate severity, defined as PRAM score of 5-8, with no other significant illnesses (lower respiratory infection, other serious infections or organic diseases) and those who have not received steroids in the last one week, were included in the study after getting written informed consent from their parents; assent was obtained if the child’s age was more than seven years.
A computer generated randomisation sequence was made and the allocation was concealed using sealed opaque envelopes. These envelopes were kept in the out-patient department and whenever an eligible child was recruited, it was sequentially opened. The children were randomised in to two groups; the principal investigator would communicate the group allotted to the pharmacist who was instructed to give the appropriate drug to the child based on the group allocated. Group A received a single dose of oral dexamethasone at the dose of 0.3 mg/kg (maximum dose 12 mg) orally stat at recruitment followed by two doses of placebo (starch powder- an innocuous and tasteless substance) 24 hours and 48 hours later. Group B received oral prednisolone at the dose of 1 mg/kg/day (maximum dose 40 mg) once a day for three days [Table/Fig-1]. So, both the groups received the intervention drug three times- zero dose at presentation, second dose and third doses after 24 hours and 48 hours of the first dose respectively. Both the intervention drugs and the placebo were made into powder and were designed to look similar in colour and texture (packed in similar looking packets).
Consort diagram.
PRAM-Paediatric Respiratory Assessment Measure
It was planned that if a child worsens after single dose of dexamethasone, then the treatment would be switched over to the existing standard of care (i.e., three day course of prednisolone) and it would be documented as treatment failure with the intervention. In case the child vomited out the medication within 30 minutes of administration, it was decided to give a second dose of the same drug; if the child vomited again within 30 minutes of the second dose, it was decided to exclude the patient from the study. Children in both groups received bronchodilator (Levosalbutamol- 0.075 mg/kg and/ or Ipratropium bromide- 250 mcg/dose given every 20 minutes for three times, then every two to four hourly as required) nebulisations as necessary and the number of such nebulisations were recorded. PRAM score which consists of measuring suprasternal and scalene muscle contraction, air entry, wheezing and oxygen saturation with a maximum score of 12, was assessed every hour until the score was <2; the children were then followed up (clinical examination for retractions, checking SpO2 when child was tachypneic with or without retractions and auscultation for wheeze) for further 48 hours to look for any relapse in wheezing before discharge.
Statistical Analysis
The time taken for PRAM score to reach a value of <2, the number of inhalational bronchodilator nebulisations required to reach the end point of PRAM score <2, and the number of relapses within 24 hours of reaching PRAM score <2 in both the groups were recorded and analysed using the student t-test.
Results
Out of 226 children who presented with acute exacerbation of asthma during the study period, 140 children had mild exacerbation and 14 had severe or life threatening exacerbation and were not eligible for the study. Of the remaining 72 children with moderate exacerbation of bronchial asthma who were eligible for the study, five children with associated lower respiratory tract infection and seven children whose parents refused to give consent were excluded and the remaining 60 children formed the study participants.
Both the groups were comparable with respect to their age, gender, baseline PRAM score at admission, number of wheeze exacerbations in the preceding year and number of children on preventive controller medications [Table/Fig-2,3]. In both the groups, upper respiratory tract infections were the most common trigger [Table/Fig-2].
Comparison of baseline characteristics in both the groups.
| Variables | Categories | Group A (Dexamethasone) | Group B (Prednisolone) | p-value(chi-square test) |
|---|
| Number of children | Percentage (%) | Number of children | Percentage (%) |
|---|
| Age (years) | 2-5 Years | 17 | 56.7% | 11 | 36.7% | χ2=2.41p-value=0.12 |
| 5-12 Years | 13 | 43.3% | 19 | 63.3% |
| Gender | Male | 17 | 56.7% | 20 | 66.7% | χ2=0.63p-value=0.43 |
| Female | 13 | 43.3% | 10 | 33.3% |
| Asthma episodes in last one year | ≤5 | 21 | 70% | 19 | 63.3% | χ2=0.3p-value=0.58 |
| >5 | 9 | 30% | 11 | 36.7% |
| Preventive therapy | Yes | 7 | 23.3% | 8 | 26.7% | χ2=0.09p-value=0.77 |
| No | 23 | 76.7% | 22 | 73.3% |
| Upper respiratory tract infections | Yes | 21 | 70% | 18 | 60% | χ2=0.66p-value=0.42 |
| No | 9 | 30% | 12 | 40% |
p-value <0.05 considered as significant
Table comparing PRAM score on admission.
| Parameter | Group A (Dexamethasone) | Group B (Prednisolone) | t-value | p-value(t-test) |
|---|
| Mean | (±)SD | Variance | Mean | (±)SD | Variance |
|---|
| PRAM Score | 5.83 | 0.83 | 0.70 | 6.13 | 0.82 | 0.67 | 1.36 | 0.09 |
p-value < 0.05 considered as significant, PRAM-Paediatric respiratory assessment measure
The mean number of bronchodilator nebulisations required for prednisolone group is 42.7±13.5 and dexamethasone group is 40.97±17.98. The mean time (in hours) required to attain PRAM score of <2 in prednisolone group is 58.6±11.5 and in dexamethasone group is 56.9±12.9 [Table/Fig-4].
Table showing comparison of outcome variables.
| Clinical parameters | Group A (Dexamethasone) | Group B (Prednisolone) | t-value | p-value(t-test) |
|---|
| Mean | (±)SD | Variance | Mean | (±)SD | Variance |
|---|
| No. of Bronchodilator nebulisations required | 40.97 | 17.98 | 323.41 | 42.73 | 13.45 | 181.17 | 0.80 | 0.21 |
| Time required for PRAM to become <2 (in hours) | 56.93 | 12.88 | 165.93 | 58.60 | 11.46 | 131.28 | 0.53 | 0.30 |
p-value < 0.05 considered as significant, PRAM-Paediatric respiratory assessment measure
The results show that there are no significant differences (p-value < 0.05 taken as significant) between dexamethasone and prednisolone groups in the outcomes measured. The study was however under-powered as evident from the wide standard deviation noticed in the results (Post-hoc power based on the mean and SD).
Discussion
This double-blinded pilot randomised controlled trial was conducted to find out whether single dose oral dexamethasone is more efficacious than three day course of prednisolone in children in the age group two to twelve years with moderate exacerbation of acute bronchial asthma.
Upper respiratory infections were the most common triggers for asthma exacerbations in both groups (70% in dexamethasone group vs 60% in prednisolone group) which confirms with the existing literature [9]. The outcome variables such as mean time (in hours) required to attain PRAM score of <2, number of bronchodilator nebulisations required showed no statistically significant differences between the groups; there were no relapses within 24 hours of reaching PRAM score <2 in both the groups.
This finding that single dose of oral dexamethasone is at least as good as three day course of prednisolone is in accordance to many previous studies [5-7,10]. The most recently published study by Cronin JJ et al., also has proven that dexamethasone is comparable to prednisolone, although the present study differed from their study in several aspects-sample size, severity of exacerbation in the included subjects and study methodology [10]. Despite the subjects being from different ethnicities, a shorter course and lesser number of doses of oral dexamethasone has been consistently proven to be effective in many other studies done in varied settings [5-7]. Our study done in South Indian children also adds to this body of evidence. The findings are also similar to other studies where intramuscularly administered dexamethasone was compared with oral prednisolone [11-13]. Thus irrespective of the route of administration, single dose dexamethasone seems to be equivalent to multiple doses of prednisolone.
There were no adverse events or treatment failure reported in the dexamethasone group. Two children in the prednisolone group vomited once whereas, none in the dexamethasone group had vomiting. Oral dexamethasone has been shown to be more palatable than prednisolone in a study done by Hamesh H et al., [14]. We did not specifically study the acceptability and palatability of the medicines using any standardised scales. The compliance to intervention was 100 % in both the groups. Though, in a study done by Lucas-Bouwman M et al., a poor compliance to multiple day course of oral prednisolone was observed due to poor palatability, we did not notice the same in our study [15].
Thus, the present study shows that a single dose dexamethasone is at least as good as three day course of prednisolone with respect to the time taken for the acute exacerbation to subside as monitored by the PRAM score in South Indian children aged two to twelve years. The strengths of our study include the design-it was a placebo controlled, double-blinded trial. Meticulous efforts were made to make the drugs in both groups to be very similar in volume, colour and texture, thereby removing any potential bias that could arise from open-labelled design. Also, we had used objective criteria for assessing the improvement i.e., the PRAM score.
Limitation
Our study has some limitations. The study was a pilot randomised controlled trial conducted on a small sample and the age group was restricted to two to twelve years. All the children needed hospitalisation for their treatment since, children with moderate severity only were included. In children with mild asthma exacerbation who would not require hospitalisation, such a comparative study on ambulatory management would be of immense use for office practice. Hence, the findings in this study need to be confirmed with a larger sample size and also in diverse settings-ambulatory basis, milder asthma exacerbations etc.
Conclusion
Our pilot study strengthens the idea for using dexamethasone in children with acute asthma exacerbations in place of prednisolone and at the same time, opens up newer avenues for future research.
p-value <0.05 considered as significant
p-value < 0.05 considered as significant, PRAM-Paediatric respiratory assessment measure
p-value < 0.05 considered as significant, PRAM-Paediatric respiratory assessment measure