Erdheim-Chester Disease: Utility of 18F-FDG Positron Emission Tomography
Angelina Cistaro1, Maria Licari2, Simone Margotti3, Daniele Penna4, Vincenzo Arena5
1 Nuclear Physician, Coordinator of PET Paediatric AIMN Study InterGroup, Positron Emission Tomography Centre, IRMET, Affidea, Turin, Italy.
2 Nuclear Physician, Department of Nuclear Medicine, ARNAS Civico Hospital, Palerm, Italy.
3 Nuclear Physician, Positron Emission Tomography Centre, IRMET, Affidea, Turin, Italy.
4 Nuclear Physician, Positron Emission Tomography Centre, IRMET, Affidea, Turin, Italy.
5 Nuclear Physician, Positron Emission Tomography Centre, IRMET, Affidea, Turin, Italy.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Angelina Cistaro, V. O. Vigliani 89/A, 10138 Turin, Italy.
E-mail: angelina.cistaro@affidea.it
Erdheim-Chester Disease (ECD) is a rare non Langerhans cell histiocytosis of unknown origin with multiorgan involvement. We report a case of a man who presented to us with haematuria, asthenia, fever, nausea and malleolar oedema. After computed tomography and magnetic resonance imaging, the patient underwent a Positron Emission Tomography/Computed Tomography (PET/CT) with 18F-fluorodeoxyglucose (18F-FDG) that revealed multiple focal uptake in the skeleton and also visceral and vascular involvement. After a retroperitoneal biopsy, diagnosis of ECD was made. The treatment begun with steroids and after one month, a second 18F-FDG PET/CT was performed, highlighting a partial response. After five months of specific treatment with inhibitors of tyrosine kinases, a third 18F-FDG PET/CT was done showing a complete response. The 18F-FDG PET/CT allows assessing the extent of involvement in ECD, to detect the most easily accessible sites for diagnostic biopsy and to monitor disease activity and response to consolidated and new therapies.
Fluorodeoxyglucose, Non langerhans cell histiocytosis, Vemurafenib
Case Report
A 48-year-old male was referred to our department to undergo an 18F-FDG PET/CT for a fever of unknown origin. There was no significant past medical history except for a suspected Paget’s disease identified by MRI scan which was performed for pain in the knees. Since one year, the patient was complaining of haematuria, asthenia, fever, nausea and vomiting of unknown origin. Laboratory tests showed a mild inflammatory status: erythrocyte sedimentation rate and C reactive protein were above normal values respectively 112 mm/1st h (nv<15) and 49.4 mg/l (nv<8) respectively. A mild alteration of renal function was also observed (creatinine 1.5 mg/dL). A brain, thoracic and abdominal contrast-enhanced CT showed bilateral renal cysts and a slight reticular thickening of the left perirenal adipose tissue and the ipsilateral gerota fascia. These findings were confirmed by a subsequent contrast-enhanced MRI of the kidney. The PET study showed bilateral, symmetric hypermetabolic lesion involving the diaphysis and metaphysis of the lower part of the femora and the upper part of tibiae. Furthermore the PET showed diffuse radiotracer uptake in both femoral arteries and in kidney’s parenchyma and in the fat of retroperitoneum around the kidney [Table/Fig-1a,b], that appeared pathologically increased at coregistration CT [Table/Fig-1c].
Imaging performed during the first hospitalisation: a) 18F-FDG PET; b) Fused PET/CT image demonstrating increased radiotracer uptake in the retroperitoneal fat tissue; c) Coronal CT showing moderate thickening of the perirenal fat tissue, particularly in the left region. The red cross indicate the moderate 18F-FDG uptake in patient affected by Erdheim Chester disease with thickening of retroperitoneal perirenal fat.
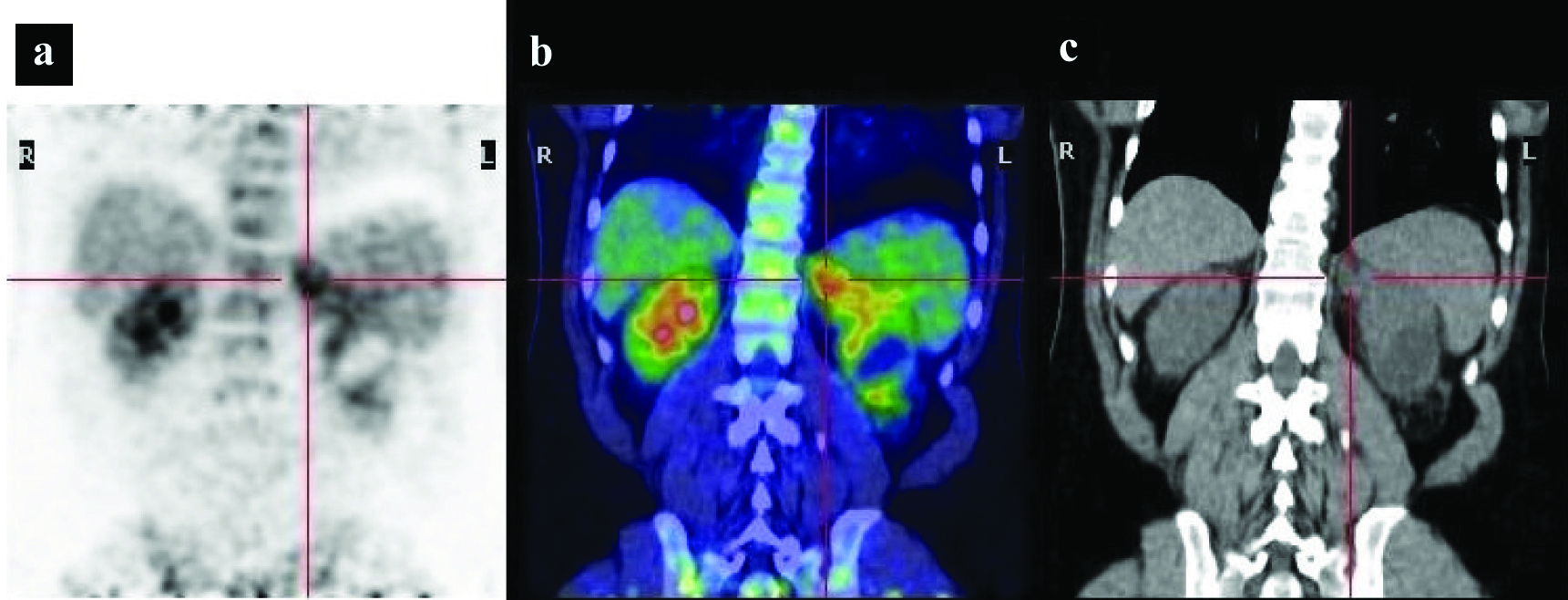
Asymmetric uptake was seen at the level of optic nerves. According to the PET/CT findings the patient was subsequently evaluated by means of an abdominal MRI, without contrast, that showed the presence of pathological tissue located around the kidneys with infiltration and stenosis of both renal arteries. Furthermore, retro-orbital and cardiac pathological tissue was detected by a further contrast enhanced MRI. A retroperitoneal 18F-FDG PET/TC guided biopsy was done on the sites of most intense FDG uptake. The histological examination revealed ECD. A genetic test for BRAF gene was also done and a mutation was detected at position 600.
The patient began treatment with corticosteroids (Prednisone 7.5 mg/day) and after one month of therapy a new 18F-FDG PET/CT study was performed in order to evaluate the response to treatment. The scan showed persistence of FDG uptakes in tibiae and kidneys and a slight reduction of radiotracer uptake intensity in femora, consistent with probable local response to pharmacological therapy.
On the basis of genetic results, of the scarce response to the conventional therapy and the multi organ involvement, especially the heart. The patient was treated with Vemurafenib (480 mg/day for six months), inhibitor of BRAF gene. After five months of therapy a third 18F-FDG PET/CT was done that showed a complete response to therapy.
Discussion
Erdheim-Chester Disease is a rare form of non Langerhans’ cell histiocytosis that affects mainly adults between their fifth and seventh decades of life with a slight male predominance [1,2]. The small number of patients and the heterogeneity of clinical manifestations are an obstacle in the understanding of this condition [3]. One-year and five-year survival rates are 96% and 68% respectively [4]. ECD is a rare and multi-systemic disorder with an unclear aetiology. Recently several, researchers demonstrated that an abnormal increase in T helper-1 immune response, producing several proinflammatory cytokines, such us interferon-α, interleukin-12, monocyte chemotactic protein-1 which are probably responsible for the recruitment and activation of the histiocytes in the tissues. The typical pattern is a polymorphic granuloma, infiltrated with CD68-positive and CD1a-negative foamy histiocytes, and fibrosis or xanthogranulomatosis [1]. BRAF-V600E mutations had been observed in 50% to 100% of ECD in terms of the diagnostic work-up, and also strongly supports that ECD is a clonally derived and RAS-RAF-MEK-ERK–driven disorder [5].
Clinically ECD is a multisystem disease. Skeletal involvement is extremely frequent (96%) therefore bone pain is the most common symptom during the course of the disease [6]. Extraskeletal involvement can involve the kidney, skin, central nervous system, lung and heart. The diagnosis of ECD is difficult and is usually based on clinical and radiological findings but always histopathological confirmation is necessary. Bilateral symmetrical long bone involvement, showed by radiologic cortical osteosclerosis typically in the diaphyseal and metaphyseal regions, is common [3]. Furthermore cardiovascular, lung and retroperitoneal infiltrating disease are other typical radiologic findings.
Bone lesions may be on X-rays, CT, MRI, and 99mTc diphosphonates bone scintigraphy. The last one may reveal osteosclerosis, in particular symmetric and abnormally strong labelling of the distal ends of the legs, and sometimes of the arms [6].
Anyway, bone lesions normally detected by radiological examination may sometimes be misinterpreted because there is a mixed pattern with lytic and sclerotic lesions, which makes difficult the differential diagnosis of Langerhans cell histiocytosis from Paget’s disease such as in our patient [7]. In this setting the use of 18F-FDG PET/CT allows to highlight disease at bone marrow level, too [7] helping in correct diagnosis and also highlighting the extra-skeletal disease. Infact, in about half of all patients, other typical findings of extraskeletal sites of disease can be observed, such as in the retro-orbital tissue, cardiac tissue, retroperitoneal space, brain and endocrine tissue. For visceral involvement, CT is most useful, while MRI imaging is more sensitive for cardiovascular lesions [7]. The advantage of 18F-FDG PET/CT scanning on other conventional imaging modality is the possibility to evaluate the full body in a single session and permitting the evaluation of the extension of ECD disease [7,8].
Arnaud L et al., in a retrospective study of 65 PET scans performed in 31 consecutive patients, reported the potential utility of FDG-PET scanning in the evaluation of osseous but also neurologic, vascular, pericardial and orbital involvement in patients with ECD [9]. The sensitivity varies among the different anatomical sites of involvement but 18F-FDG PET/CT scan shows excellent specificity when compared with other conventional imaging modalities [10].
An 18F-FDG PET/CT may also assist to identify candidate areas for CT guided percutaneous biopsy when extra-skeletal involvement seems prominent and/or a discrete mass may not be present in conventional imaging modalities [3].
To date, the first therapeutic approach for ECD consist of cortisteroids administration but novel treatment approaches are coming based on immunosuppressants such as interferon α and sirolimus, monoclonal antibodies such as tocilizumab and inhibitors of tyrosine kinases such as vemurafenib. The last one showed promising results in systemic disease form due to its capacity to inhibit the serine-threonine kinase, blocking the uncontrolled cell proliferation caused by V600e mutations in the BRAF gene [11].
Because of its ability to quantify the rate of FDG uptakes, PET is a powerful modality for monitoring disease activity and response to therapy during the course of patient treatment either to appreciate treatment efficiency or decide treatment modification [7].
Our case confirms the role of 18F-FDG PET/CT in identifying the extension of the disease and in selecting the sites of biopsy. The present case suggests the 18F-FDG PET/CT utility in the assessment of response to consolidated or new treatments, highlighting the lack of effectiveness of corticosteroid therapy and the complete response to the therapy with inhibitors of BRAF gene.
Conclusion
Our case shows the impact of 18F-FDG PET/CT in patients affected by ECD with the main benefit of simultaneously evaluating the extent and the disease activity both on skeletal and extraskeletal sites. 18F-FDG PET/CT may also play an important role in identifying the site of biopsy. Because of its ability to quantify the FDG uptake, PET/CT may prove to be a powerful modality for monitoring disease activity and response to consolidated and new therapies.
[1]. Haroche J, Arnaud L, Cohen-Aubart F, Hervier B, Charlotte F, Emile J, Erdheim-Chester diseaseCurrent Rheumatology Reports 2014 16(4):41210.1007/s11926-014-0412-024532298 [Google Scholar] [CrossRef] [PubMed]
[2]. Volpicelli ER, Doyle L, Annes JP, Murray MF, Jacobsen E, Murphy GF, Erdheim-Chester disease presenting with cutaneous involvement: a case report and literature reviewJournal of Cutaneous Pathology 2011 38(3):280-85.10.1111/j.1600-0560.2010.01650.x21143617 [Google Scholar] [CrossRef] [PubMed]
[3]. Mazor RD, Manevich-Mazor M, Shoenfeld Y, Erdheim-Chester Disease: A comprehensive review of the literatureOrphanet Journal of Rare Diseases 2013 8:13710.1186/1750-1172-8-13724011030 [Google Scholar] [CrossRef] [PubMed]
[4]. Arnaud L, Hervier B, Neel A, Hamidou MA, Kahn JE, Wechsler B, CNS involvement and treatment with interferon-alpha are independent prognostic factors in Erdheim-Chester disease: a multicenter survival analysis of 53 patientsBlood 2011 117(10):2778-82.10.1182/blood-2010-06-29410821239701 [Google Scholar] [CrossRef] [PubMed]
[5]. Haroche J, Charlotte F, Arnaud L, von Deimling A, Hélias-Rodzewicz Z, Hervier B, High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytosesBlood 2012 120(13):2700-03.10.1182/blood-2012-05-43014022879539 [Google Scholar] [CrossRef] [PubMed]
[6]. Haroche J, Amoura Z, Dion E, Wechsler B, Costedoat-Chalumeau N, Cacoub P, Cardiovascular involvement, an overlooked feature of Erdheim-Chester disease: report of 6 new cases and a literature reviewMedicine 2004 83(6):371-92.10.1097/01.md.0000145368.17934.9115525849 [Google Scholar] [CrossRef] [PubMed]
[7]. Antunes C, Graca B, Donato P, Thoracic, abdominal and musculoskeletal involvement in Erdheim-Chester disease: CT, MR and PET imaging findingsInsights into Imaging 2014 5(4):473-82.10.1007/s13244-014-0331-725017251 [Google Scholar] [CrossRef] [PubMed]
[8]. Cistaro A, Cucinotta M, Cassalia L, Priola A, Priola S, Pappalardo 18F-FDG PET/CT, cytoreductive surgery and intraperitoneal chemohyperthermia for the therapeutic management in peritoneal carcinomatosis: A pilot studyRevista Espanola De Medicina Nuclear E Imagen Molecular 2016 35(4):232-37.10.1016/j.remn.2016.01.00126907833 [Google Scholar] [CrossRef] [PubMed]
[9]. Arnaud L, Malek Z, Archambaud F, Kas A, Toledano D, Drier A, 18F-fluorodeoxyglucose-positron emission tomography scanning is more useful in followup than in the initial assessment of patients with Erdheim-Chester diseaseArthritis and Rheumatism 2009 60(10):3128-38.10.1002/art.2484819790052 [Google Scholar] [CrossRef] [PubMed]
[10]. Haroche J, Amoura Z, Wechsler B, Veyssier-Belot C, Charlotte F, Piette JC, [Erdheim-Chester disease]Presse Medicale 2007 36(11 Pt 2):1663-68.10.1016/j.lpm.2007.04.03217618076 [Google Scholar] [CrossRef] [PubMed]
[11]. Haroche J, Cohen-Aubart F, Emile JF, Maksud P, Drier A, Tolédano D, Reproducible and sustained efficacy of targeted therapy with vemurafenib in patients with BRAF(V600E)-mutated Erdheim-Chester diseaseJournal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 2015 33(5):411-8.10.1200/JCO.2014.57.195025422482 [Google Scholar] [CrossRef] [PubMed]