The small size and ease of placement of MSIs have opened up a new field of possibilities for orthodontic treatment with the concept of absolute anchorage. However, many orthodontists are apprehensive about their placement procedures as the limiting factor is the proximity of root structures. Even if the clinician takes utmost care while placing the implant, there is still a possibility that the implants may damage the roots.
The literature related to this is largely limited to the case reports or case-series studies and mostly involved drilled screws. Evidences are present on the root damage caused by errantly placed trans-alveolar screws and correlated damage to the pulpal vitality and root damage to premolars secondary to inter-maxillary fixation screw placement [1-3]. Interpretation of these results is difficult because vitality testing of traumatised teeth is unreliable.
Even though, many studies have been carried out on animals to study the repair of roots after intentional damage, the histological evidence of this repair process in humans is still lacking [4-7]. The scarcity of evidence-based literature about the management of MSIs induced traumatic root injuries compromises the clinician’s ability to provide proper informed consent to the patient. Hence, it is important to know the histological response secondary to mini-screw induced root damage. Thus, the aim of this study was to evaluate histologically the time period required for root repair after damage due to intentional contact with orthodontic MSIs.
Materials and Methods
The present study was observational type of study and was carried out in the Department of Orthodontics of Dr. D.Y. Patil Dental College and Hospital, Pune, Maharashtra, India for duration of one year from January 2012 to December 2012. Patients who reported to the department at the institute were screened for the study. Ten subjects were selected for the study. Sample size was calculated based on convenient sampling technique. The inclusion criterion included only those patients in the age group of 18 to 45 years, who required fixed appliance therapy in both the arches with first premolar extraction indicated as part of their treatment. Periodontally compromised patients or patients with any systemic disease were excluded from the study. The institutional research ethics committee approved the study design. Ten patients were pre-bonded with Pre-adjusted Edge wise Appliance System. The implants were of self-drilling design having a diameter of 1.6 mm and length of 8 mm (Denticon).
The patients were explained about the procedure prior to the extractions and their informed consent was taken. All sterilisation and disinfection protocols were followed. The position of the roots of the four premolars to be extracted was clinically determined for each of the patient. Local anaesthesia was given.
Then the MSIs were removed from their sterile packets and were placed in such a way that there was direct contact with the buccal root surfaces of first premolars which were to be extracted for the purpose of orthodontic treatment [Table/Fig-1a]. Position for placement of implants was standardised by measuring the distance on a graduated probe from the center of the bracket previously bonded on the premolars [Table/Fig-1b]. Implant was placed along the long axis of the tooth on the buccal side [Table/Fig-1c]. Resistance and tactile perception were the criteria to determine the contact with the root surface. Six complete turns were given after initial contact was achieved. This procedure was followed for each premolar to be extracted in every patient.
a) Standardisation of distance for implant placement; b) Marking the point for implant placement; c) Implant placement at the marked point; d) Damage is confirmed on the buccal side of the premolar (marked with red circle).
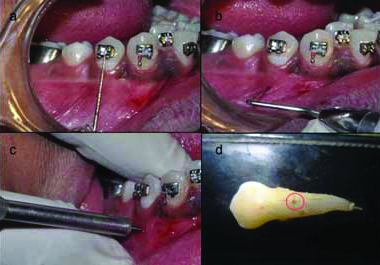
Micro-screw implants were removed from the patient’s mouth immediately after the above-mentioned procedure was done. The premolar of the upper right quadrant was extracted on the next day. The premolar of the upper left quadrant was extracted after three weeks, premolar of lower left quadrant was extracted after six weeks and the premolar of lower right quadrant was extracted after 12 weeks.
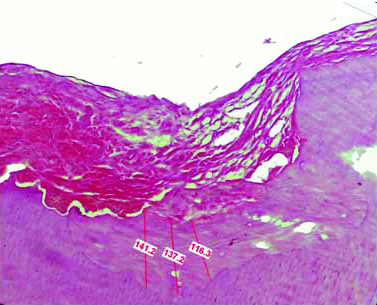
The extracted premolars were transferred to a bottle containing 10% formalin to carry out the further histological procedures. The damage made was confirmed by visual evaluation after extraction on the buccal side of the premolar root [Table/Fig-1d]. The extracted tooth was suspended in 5% nitric acid for 10 to 12 days and was then tested for complete decalcification. The specimen was dehydrated by placing it in increasing percentages of alcohol and was then embedded into paraffin block to make serial longitudinal sections of 3 μm thickness. The prepared sections were stained with H&E and were observed under magnification of 5X, 10X and 40X. For measuring the thickness of the newly formed cementum, the stained slides were observed under research microscope with image analysing software (Leica QWin) and were marked at three different points to calculate a mean thickness value [Table/Fig-2]. The 40 samples were divided into four groups as in [Table/Fig-3].
Measurement of cementum thickness after 12 weeks with leica QWin software.
H&E stain 400X magnification
Different groups on the basis of time of extraction after damage
| Groups on the basis of time of extraction after damage by MSIs to the root |
|---|
| Group | Time of extraction after damage | Sample size |
|---|
| A | 1 day | 10 |
| B | 3 weeks | 10 |
| C | 6 weeks | 10 |
| D | 12 weeks | 10 |
Statistical Analysis
Graphpad Prism software (version 6) was used for the statistical analysis. Comparison of mean healing thickness and standard error of mean in each group was done with one-way analysis of variance (ANOVA). Students t-test was used to determine the statistical significance of the difference between different groups.
Results
Subjects in the study did not complain of undue pain after the trauma. It was evident from the histological evaluation that the dentin in all specimens was damaged although, the quanta of damage and repair were variable. No evidence of root resorption or ankylosis as evidenced by extraction of the teeth was seen.
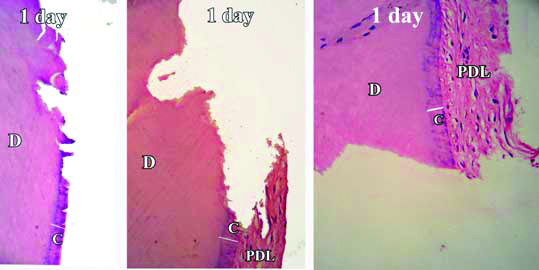
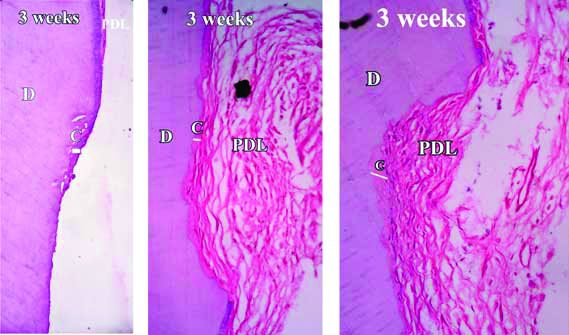
In Group-A, the stained demineralised sections showed that root damage had occurred in all the samples. The damaged area showed breach in the continuity of cementum. Absence of basophilic cementoblasts was a concurrent finding throughout the Group-A images [Table/Fig-4]. In Group-B, beginning of cementum formation was noted. A thin basophilic lining of cementum formation was observed around the damaged area [Table/Fig-5]. The average mean thickness found at the end of three weeks was about 8.49 μm. In Group-C, healing of root cementum was found to have significantly increased at the end of six weeks [Table/Fig-6]. The damage was visible but was lesser as compared to Group-B. Cementum formation was not only limited to the marginal area but had also extended up to the center of damaged area. The average mean thickness found at the end of six weeks was about 69.05 μm. In Group-D, almost complete cementum deposition was found at the end of twelve weeks [Table/Fig-7] and the average mean thickness was found to be 192.4 μm. Further, the mean values of repaired cemental thickness between the four groups and their comparison showed significant differences [Table/Fig-8,9].
Demineralised section of root showing damage and break in continuity of cementum after one day. Damage area is seen as the notch extending up to dentin.
H&E stain with 50X, 100X and 400X magnification
Demineralised section of root showing healing and cementum formation after three weeks (seen as basophilic lining in the area of damage).
H&E stain with 50X, 100X and 400X magnification
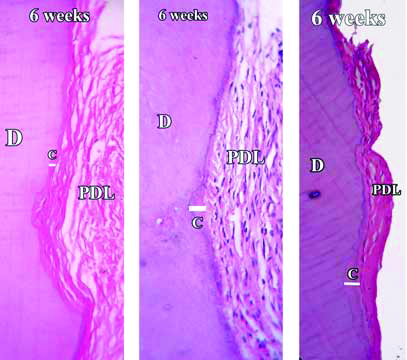
Demineralised section of root showing healing and cementum formation after six weeks.
H&E stain with 50X, 100X and 400X magnification
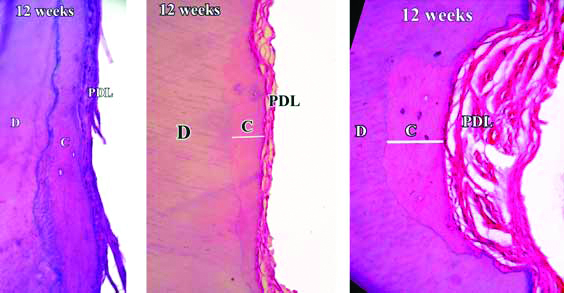
Demineralised section of root showing healing and cementum formation after 12 weeks. Basophilic cementoblasts are seen at the area of damage.
H&E stain with 50X, 100X and 400X magnification
Thickness of newly formed cementum (in μm) for each sample and comparison of mean thickness between different groups.
| Sr No | Group A(1 Day) | Group B(3 Weeks) | Group C(6 Weeks) | Group D(12 Weeks) |
|---|
| 1. | 0 | 9.400 | 65.766 | 131.566 |
| 2. | 0 | 8.433 | 50.333 | 257.700 |
| 3. | 0 | 7.466 | 81.333 | 188.133 |
| 4. | 0 | 11.066 | 76.900 | 214.466 |
| 5. | 0 | 8.166 | 65.233 | 199.600 |
| 6. | 0 | 7.133 | 76.900 | 178.566 |
| 7. | 0 | 9.566 | 66.366 | 206.666 |
| 8. | 0 | 7.666 | 70.166 | 166.700 |
| 9. | 0 | 8.066 | 72.066 | 183.266 |
| 10. | 0 | 7.966 | 65.400 | 196.633 |
| Mean±Sem | 0.0±0.0 | 8.49±0.37 | 69.05±2.75 | 192.40±10.37 |
(SEM: Standard Error of Mean)
Differences in the mean cemental repair (μm) during the study.
| Comparison Between Groups | Mean±Sem(Cementalrepair) | t-value | p-value |
|---|
| Group A | 0.0±0.0 | 22.56 | <0.001 |
| Group B | 8.493±0.37 | | |
| Group A | 0.0±0.0 | 25.11 | <0.001 |
| Group C | 69.05±2.75 | | |
| Group A | 0.0±0.0 | 18.54 | <0.001 |
| Group D | 192.4±10.37 | | |
| Group B | 8.493±0.37 | 21.82 | <0.001 |
| Group C | 69.05±2.75 | | |
| Group B | 8.493±0.37 | 17.71 | <0.001 |
| Group D | 192.4±10.37 | | |
| Group C | 69.05±2.75 | 11.49 | <0.001 |
| Group D | 192.4±10.37 | | |
(SEM: Standard Error of Mean)
Discussion
Mini-screws should be placed into the alveolar bone without any risk of damage to the adjacent roots [1]. Limiting factors for placement of the MSI include the proximity of root structures leading to unwarranted contact and the angulations needed for anchorage control. Asscherickx K et al., published the most recent controlled study in which histological examination of three teeth damaged secondarily to MSI placement showed initial repair of the periodontal structures in 12 weeks after removal of the screws with healing nearly complete after 20 weeks [8]. The study provides a basis for the time period in which repair of the root defects occurs.
In the present study, decalcification method was preferred over using ground section. During ground section preparation, initially formed cementum (cementoid), which is organic in nature gets removed. Therefore, it is not possible to measure the correct thickness of newly formed cementum. Since, cementum contains 50-55% organic matrix, it is possible to measure the existing as well as newly formed cementum using decalcified sections.
The study used greater diameter implants (1.6 mm) to ensure enough damage to the root surface in order to study the healing process histologically using a decalcified section. Moreover, the initial bur hole in cortical bone made the placement of implants easy preserving the sharpness of the implant threads. Measuring the distance on a graduated probe from the center of the bracket and maintaining the same distance for all the teeth in that patient determined the position for placement of implant. The tactile resistances of the MSIs after touching the root surface was also a fair indicator to assure that the roots were damaged.
The MSIs were placed under local anaesthesia and were removed immediately under the same anaesthesia causing minimum pain and discomfort to the patient. Along the study duration, no patient reported with any further discomfort, pain or inflammation around the area of damage, which indicated that the damage was confined to the root dentin and not to the pulp.
Repair of cementum after intentional injuries with a temporary skeletal anchorage device has been studied qualitatively in beagle dogs by several authors [5-7]. There has been only one human study so far showing quantitative measurements of reparative cementum histologically after intentional contact with MSIs [9]. Kadioglu O et al., had previously done a similar evaluation of healing of roots after intentional damage with MSIs in humans with the help of SEM [10]. This human study further evaluates the repair process of root after intentional damage using histological method.
Stained decalcified sections were examined under binocular compound microscope. All the specimens revealed favourable healing response except day one samples. The day one specimens revealed a distinct indentation of the cementum layer of the tooth and showed no evidence of cementum formation [Table/Fig-4]. The three week specimens showed thin eosinophilic band of cementum formation of variable thickness and was devoid of cementocytes and incremental lines of salters [Table/Fig-5]. A study by Kasim SA et al., showed significant repair at the end of four weeks [9]. However, the results of present study show that significant root repair takes place early as well, i.e., at the end of three weeks. The six weeks specimens revealed a thicker eosinophilic band of cementum formation, occasional incremental lines of salter and cementocytes [Table/Fig-6]. The repair process seemed to have proceeded to involve the defect area, but not completely. The 12 weeks specimen showed greater thickness with frequent evidence of incremental lines of salters and cementocytes [Table/Fig-7]. The repair had proceeded to involve the entire defect area. These results were similar to the study by Hellden L [11]. He proposed a timeline for humans after root damage with the appearance of cementoid tissue after approximately 25 days and cellular cementum by 40 days. Teeth extracted after two to three months showed Periodontal Ligament (PDL) fibers growing into the newly formed reparative cementum.
At many places single cell lining was seen adjacent to external cemental surface possibly suggesting cementoblastic activity. All the specimens except day one specimens displayed tightly adhering PDL comprising of collagen fibers and fibroblasts activity. This suggests that the normal repair was in progress characterised by regeneration of the periodontal ligament and a new layer of cementum evident at the end of 21 days. These results do not match with the study by Hellden L who reported first evidence of cementum deposition after 25 days [11].
The rate of healing was progressive and significant between all the groups especially between six weeks and 12 weeks. The mean average thickness of cementum formed was 8.4 μm at the end of three weeks, 69.05 μm at the end of six weeks and 192.5 μm at the end of 12 weeks. [Table/Fig-8] This increased thickness of cementum in the damaged area can be attributed to the damage in that area and not to the normal thickness of cementum. The repair process observed in this study can be categorised as anatomic repair as the notch created by the damage was eventually repaired to re-establish the former outline of the root surface.
The time duration protocol used in this study was different than in other human studies. Kadioglu O et al., used duration of four and eight weeks to evaluate healing by SEM [10], while study done by Kasim SA et al., used a time period of four, eight and 12 weeks to evaluate healing using histological method [9]. Although, both these studies showed the beginning of cementum repair along damaged site as early as four weeks, this study showed cementum formation by the end of three weeks. Previously a counting grid was used to evaluate the percentage of repair at various time intervals. However, present study used the Leica QWin software for measurement that provides further in detail the exact quantitative measurement of thickness of newly formed cementum in μm.
The study shows that even if iatrogenic damage is made, immediate removal of the implant will allow complete healing of the damaged root by the end of 12 weeks. The study provides the clinicians with an evidence of significant root repair between three weeks to 12 weeks after intentional damage with MSIs in humans. This evidence can be useful in allowing a healing period of 12 weeks in cases of iatrogenic damage and to have a proper informed consent.
Limitation
The findings of this study are limited by a small sample size. Further, studies with larger sample size are warranted to fully investigate the histological effects of damage from mini-screws on root surface morphology as well as the effects on the pulp tissue in humans.
Conclusion
The intentionally damaged root surfaces in this study showed a progressive repair and healing within 12 weeks. The root repair was nearly complete at the end of 12 weeks as the notch created by intentional damage was healed. Thus, in case, if the root is damaged due to improper placement technique or tipping of the micro-screw due to orthodontic loading, a minimum healing period of 12 weeks is advocated during which no further tooth movement should be carried out.
(SEM: Standard Error of Mean)
(SEM: Standard Error of Mean)