The VCP is a relatively common clinical finding, sometimes the only sign of underlying sinister disease [1-7]. Varied numbers of aetiologies have been implicated including vascular, neoplastic infiltration, surgical/iatrogenic, inflammatory, infectious and traumatic causes [4,8-12]. However, substantial number of cases may be idiopathic [4,8-10,12-13]. Any lesion along the course of vagus nerve before branching of Recurrent Laryngeal Nerve (RLN) or involving RLN itself may result in VCP [2,13-16]. Unilateral VCP is more common, although bilateral involvement can also be seen [13]. Due to its long anatomical course left RLN is more vulnerable and thus left VCP is reported 1.4 to 2.5 times more frequently than right VCP [1,7,11,13,15-17].
Despite the fact that nearly half of the cases of VCP are idiopathic, the imaging studies are still indispensible for evaluating the varied aetiologies [3]. The CT sections from the skull base upto Aortopulmonary (AP) window can be a valuable diagnostic tool and helpful in guiding early specialist referral, suggesting further imaging and guiding for appropriate treatment [3-5,18-19].
Vocal cord palsy clinically presents as hoarseness of voice, dysphonia, breathlessness and aspiration [1,8,13]. However, some cases may be even asymptomatic. The routine CT neck imaging can reliably suggest the presence of VCP even in such cases [4,13].
With this background, in the present study the authors tried to find out, whether the evaluation of multiplanar coronal reformatted images offers any additional diagnostic advantage in the cases of unilateral VCP. In addition, we also tried to find out the consistent and reliable signs of unilateral VCP on axial CT sections.
Materials and Methods
This retrospective study comprised of evaluation of CT imaging of patients with clinically suspected VCP who were referred for CT neck and thorax in Department of Radiology in a tertiary care hospital in the previous 36 months (July 2014 to June 2017). The institutional ethical clearance was obtained. Only the cases with laryngoscopically confirmed unilateral VCP were included. The cases with laryngeal malignancy resulting in secondary cord fixation and immobilisation were excluded. We use 128 slice Philips Ingenuity multi detector CT scanner for imaging. Both pre and post contrast enhanced studies were performed for all the patients with scan done from the level of skull base upto AP window. The CT parameters were as follows: contiguous 3 mm axial sections with 3 mm increment, 0.9 mm reconstruction with pitch of 0.89, field of view 250 mm, collimation 64x0.625, acquisition time 7.3s, 120 KV and 150 mAs. Three dimensional coronal and saggital multiplanar reconstructions were done after axial acquisition. Two Radiologists (one with working experience of five years and other with working experience of more than 10 years) who were blinded for the clinical details reviewed each and every case. Following imaging signs of VCP as described by Landman GH in Laryngoscopic studies on both axial and multiplanar coronal reformatted CT images were evaluated [9,14,21]:
Thickening and medial positioning of ipsilateral aryepiglottic fold
Dilatation of ipsilateral pyriform sinus
Dilatation of ipsilateral laryngeal ventricle
Anteromedial displacement of ipsilateral arytenoid cartilage
Dilatation of ipsilateral vallecula
Fullness of ipsilateral vocal cord
Fullness of ipsilateral pharyngeal wall
Fullness of ipsilateral subglottis (evaluated on axial images)
Flattening of ipsilateral subglottic arch (evaluated only on coronal reformatted images)
In addition, we also tried to evaluate the relatively newer signs of VCP on axial CT sections i.e., ‘sail sign’ and ‘mushroom sign’ [4]. Anteromedial displacement of the ipsilateral arytenoid cartilage and medialisation of posterior cord margin combined with ipsilateral distension of laryngeal ventricle due to thyroarytenoid muscle atrophy leads to residual airway taking a shape identical to sail of a ship which is referred to as sail sign [Table/Fig-1]. Mushroom sign was identified on oblique axial CT cuts at the level of true cords and results from subglottic air anterior and contralateral to distended laryngeal ventricle on side of involvement [Table/Fig-2] [4].
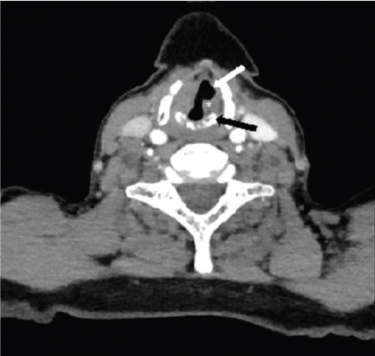
Sail sign: Axial CECT section in a 62 year old female with left RLN palsy at the level of vocal cords shows medialisation of left posterior cord margin (*), anteromedial positioning of left arytenoid cartilage (black arrow) and dilatation of left laryngeal ventricle (white arrow) giving typical sail sign.
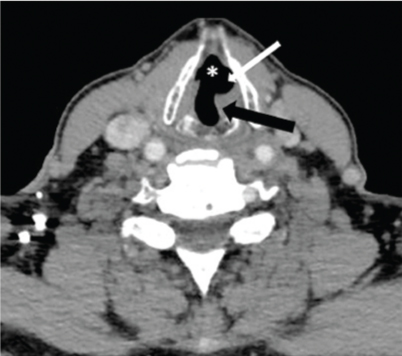
Mushroom sign: Axial CECT section in a 50 year old male with left RLN palsy shows ‘mushroom sign’ resulting from the combination of medialisation of left posterior cord margin (black arrow), dilatation of left laryngeal ventricle (white arrow) and contralateral subglottic air anteriorly (*).
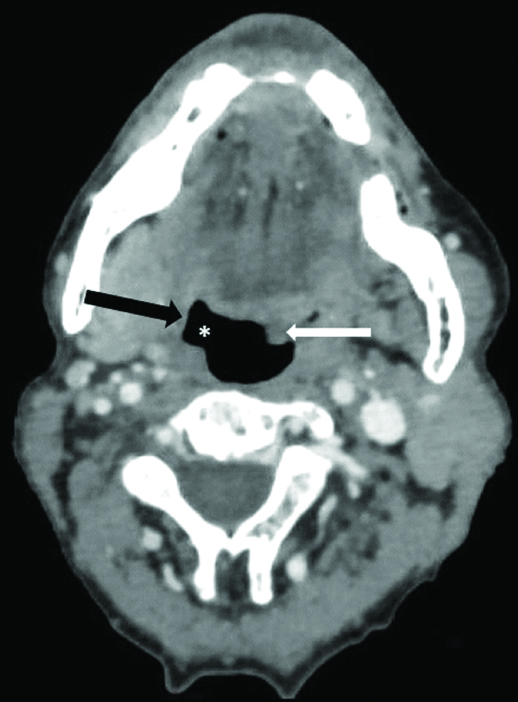
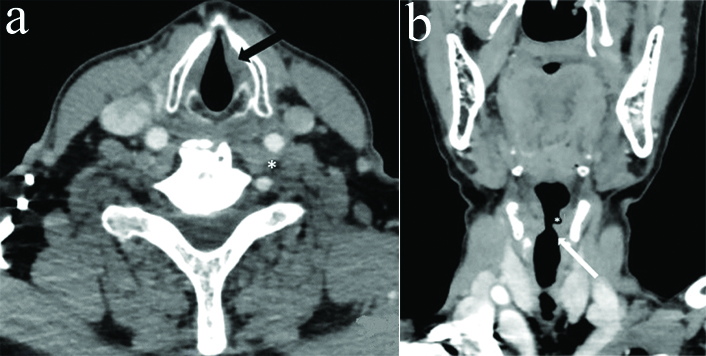
The coronal reformatted images were specifically evaluated for flattening of ipsilateral subglottic arch. Based on all above imaging signs, we tried to lateralise VCP. The presence of any offending mass/lesion in the skull base, neck or superior mediastinum along the course of vagus nerve/RLN was also assessed which may result in VCP. In cases where no causative lesion was identified, attempt was made to differentiate between proximal vagal neuropathy and isolated distal recurrent laryngeal neuropathy. Pharyngeal plexus involvement (central vagal neuropathy) was indicated by additional signs, namely bulging of ipsilateral oropharynx, thinning and atrophy of ipsilateral pharyngeal constrictor muscles and contralateral deviation of uvula [Table/Fig-3] [4,9,14].
Axial CECT section in a 65 year old male with VCP related to right central (proximal) vagal neuropathy shows bulging of right oropharynx (*), thinning of pharyngeal constrictor muscles on right side (black arrow) and deviation of uvula towards left side (white arrow).
Any difference in opinion was resolved by common consensus among both radiologists. The findings of imaging assessment were later compared and confirmed with clinical and laryngoscopic findings.
Statistical Analysis
All statistical tests were conducted using SPSS for Windows version 21.0 (SPSS Inc., Chicago, IL). Spearman’s rho (ρ) correlation coefficient was calculated to assess correlations between most frequent axial CT signs individually and also with flattening of ipsilateral subglottic arch (evaluated on coronal reformatted images) for diagnosing unilateral VCP. The strength of the absolute correlation value ‘ρ’ was determined as follows:
0.00-.19 very weak
0.20-.39 weak
0.40-.59 moderate
0.60-.79 strong
0.80-1.0 very strong
Results
A total of 41 patients were evaluated for the study, out of which 10 were excluded where vocal cords were immobilised secondary to laryngeal tumoural infiltration. These 10 cases consisted of two cases of pyriform sinus malignancy, one case of transglottic malignancy, three cases of supraglottic/glottis malignancy, two cases of glottis malignancy and two cases of glottis/subglottic malignancy.
Remaining 31 cases of unilateral VCP who met inclusion criteria were evaluated in this study. Most of the patients were elderly from 6 to 8th decade. The age of youngest patient was 36 years and of oldest was 85 years. In the present study, 21 (67.7%) patients were male and 10 (32.3%) were female. The male:female ratio was 2.1:1 The mean±SD age was 64.22±11.89 years. Nine patients (29%) had palsy on right side while 22 (71%) had palsy on left side. The ipsilaterality of VCP was diagnosed correctly on CT in all 31 cases. Three (9.7%) patients had signs of central vagal neuropathy while remaining had isolated recurrent laryngeal neuropathy. A concurrent lesion was identified along the course of RLN in 11 (35.5%) cases while remaining were idiopathic [Table/Fig-4].
Distribution of unilateral VCP in patients according to the sex, side, location and aetiology.
| Sex distribution | Males | Females | Total cases |
|---|
| Number (%) | 21 (67.7%) | 10 (32.3%) | 31 |
| Side distribution | Right | Left |
| 9 (29%) | 22 (71%) |
| Location | Central | Peripheral |
| 3 (9.7%) | 28 (90.3%) |
| Aetiology | Idiopathic | Secondary |
| 20 (64.5%) | 11 (35.5%) |
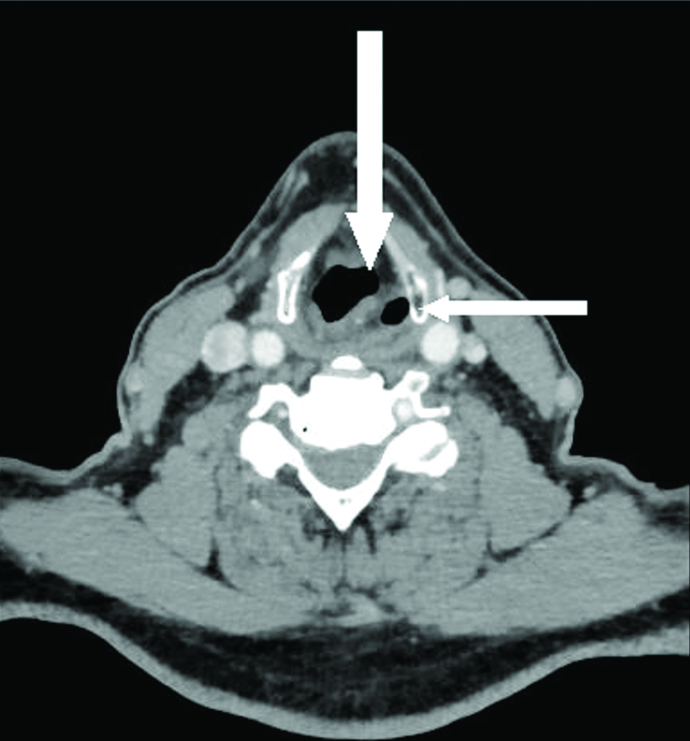
Regarding specific MDCT signs of VCP [Table/Fig-5], thickening and medial positioning of ipsilateral aryepiglottic fold was present in all the patients. Dilatation of ipsilateral pyriform sinus was seen in 27 (87%) patients [Table/Fig-6]. Dilatation of ipsilateral laryngeal ventricle was found in 29 (93.5%) patients. Anteromedial displacement of ipsilateral arytenoid cartilage was observed in 18 (58%) patients [Table/Fig-1]. Dilation of ipsilateral vallecula was seen in 7 (22.5%) cases [Table/Fig-7a]. Ipsilateral pharyngeal wall fullness was present in only 5 (16%) cases [Table/Fig-7a,b] while Ipsilateral vocal cord fullness was observed in 11 (35%) cases [Table/Fig-7c]. Ipsilateral subglottic fullness (seen on axial sections) and flattening of ipsilateral subglottic arch (evaluated on coronal reformatted images) were noted in 8 (26%) patients [Table/Fig-8a,b]. Sail sign was observed in 58% (18 out of 31) cases while mushroom sign was seen in 48% (15 out of 31) cases [Table/Fig-1,2].
Distribution of various imaging signs of unilateral vocal cord palsy in patients on MDCT assessment.
| S.No. | CT findings | Present | Absent | Total cases |
|---|
| 1 | Thickening and medial positioning of ipsilateral aryepiglottic fold | 31 (100%) | Nil | 31 |
| 2 | Dilatation of ipsilateral pyriform sinus | 27 (87%) | 4 (13%) |
| 3 | Dilatation of ipsilateral laryngeal ventricle | 29 (93.5%) | 2 (6.5%) |
| 4 | Antero-medial displacement of ipsilateral arytenoid cartilage | 18 (58%) | 13 (42%) |
| 5 | Dilatation of ipsilateral vallecula | 7 (22.5%) | 24 (77.5%) |
| 6 | Fullness of ipsilateral vocal cord | 11 (35%) | 20 (65%) |
| 7 | Fullness of ipsilateral pharyngeal wall | 5 (16%) | 26 (84%) |
| 8 | Fullness of ipsilateral subglottis(evaluated on axial images) | 8 (26%) | 23 (74%) |
| 9 | Flattening of ipsilateral subglottic arch (evaluated on coronal reformatted images) | 8 (26%) | 23 (74%) |
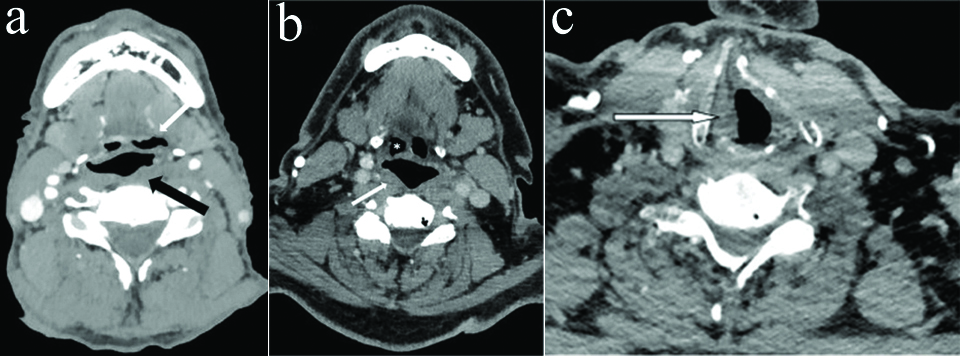
Axial CECT section in a 58 year old male with left RLN palsy at the level of hypopharynx shows thickening and medial positioning of left aryepiglottic fold (thick white arrow) and dilatation of left pyriform sinus (thin white arrow).
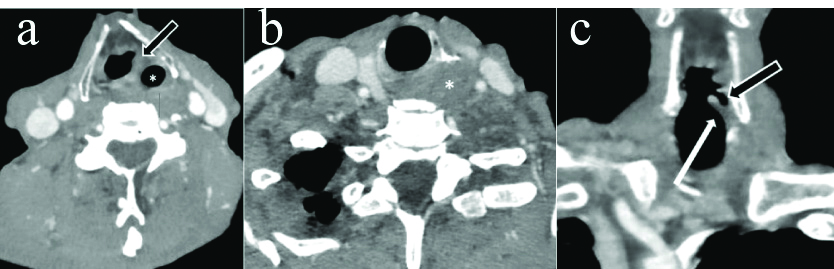
a) Axial CECT section in a 72 year old male with left RLN palsy shows dilatation of left vallecula (white arrow) and fullness of left pharyngeal wall (black arrow) b) Axial CECT section in another 74 year old male with right RLN palsy show fullness of right pharyngeal wall (white arrow) without any vallecular dilatation (*) c) Axial CECT section in same patient as in b at the level of cricoarytenoid joint shows fullness of right vocal cord.
a) Axial CECT section in a 60 year old female with left RLN palsy shows fullness of left subglottis (black arrow) b) Coronal reformatted images in same patient show flattening of subglottic arch on left side (white arrow) with dilatation of left laryngeal ventricle (*).
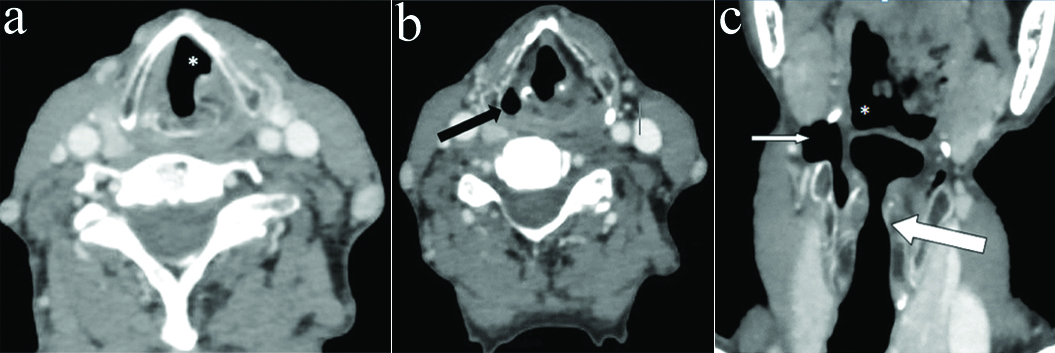
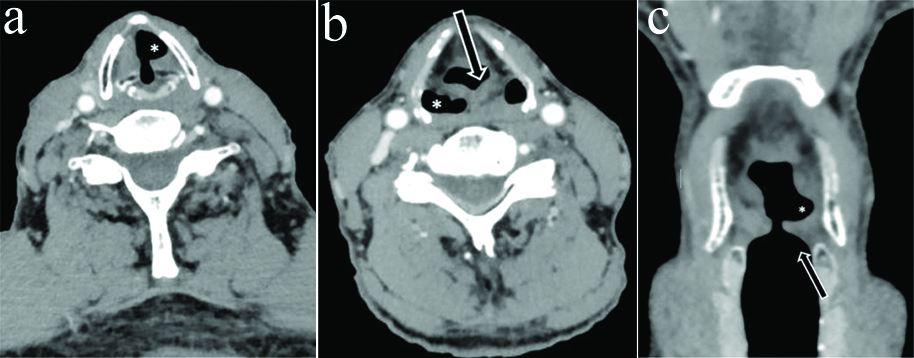
Four cases had pyriform sinus dilatation on contralateral rather than ipsilateral side out of which one case had concurrent contralateral vallecular dilatation also. Flattening of Ipsilateral subglottic arch was not found in any of these cases [Table/Fig-9,10,11 and 12].
a) Axial CECT section in a 55 year old male with left RLN palsy at the level of vocal cords shows typical sail sign (*) b) Axial section in same patient show dilatation of contralateral rather than ipsilateral pyriform sinus creating confusion (black arrow) c) Coronal reformatted images showed maintained subglottic arch on left side (thick white arrow) inspite of left VCP. In addition, dilatation of contralateral vallecula (*) and pyriform sinus (thin white arrow) is also seen.
a) Axial CECT section in an 80 year old female with left RLN palsy at the level of vocal cords shows typical sail sign (*) b) Section at the level of hypopharynx show thickening and medialisation of left aryepiglottic fold (arrow) with contralateral rather than ipsilateral dilatation of pyriform sinus (*) c) Coronal reformatted images showed dilated left lateral ventricle (*) but maintained subglottic arch on left side (arrow) inspite of left VCP.
a) Axial CECT section in a 82 year old male with left RLN palsy at the level of vocal cords shows typical sail sign (*). However, there is contralateral rather than ipsilateral dilatation of pyriform sinus (black arrow) b) Coronal reformatted images showed dilated right pyriform sinus (*) and maintained subglottic arch on left side (black arrow) inspite of left VCP.
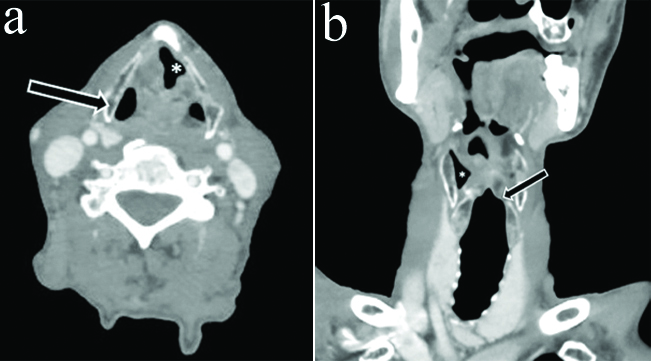
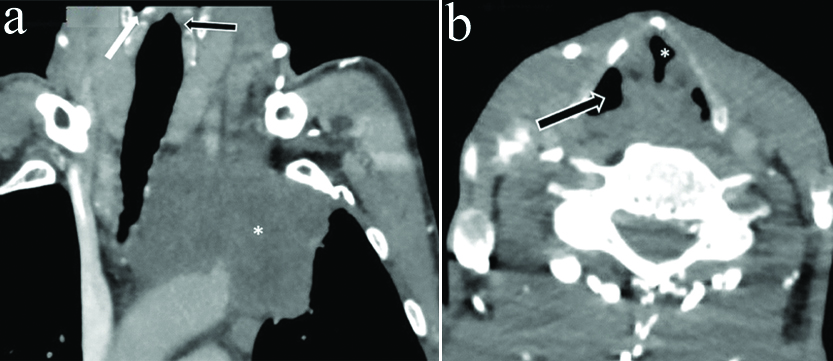
a) Coronal CECT images of neck and upper thorax in a 43 year old male with left VCP show superior sulcus tumor with mediastinal invasion (*) In addition dilated contralateral rather than ipsilateral pyriform sinus is seen (white arrow) with maintained subglottic arch on left side (black arrow) inspite of left VCP b) Axial CECT section in same patient at the level of vocal cords shows typical sail sign (*) with contralateral rather than ipsilateral dilatation of pyriform sinus (arrow).
Dilatation of Ipsilateral laryngeal ventricle and ipsilateral pyriform sinus showed very strong and statistically significant correlation for accurately diagnosing unilateral VCP. However, flattening of ipsilateral subglottic arch and dilatation of ipsilateral pyriform sinus correlated weakly for diagnosing unilateral VCP. Similarly, very weak correlation was observed between flattening of ipsilateral subglottic arch and dilatation of ipsilateral laryngeal ventricle for predicting VCP [Table/Fig-13].
Calculation of Spearman’s Rho (ρ) correlation coefficient between flattening of ipsilateral subglottic arch (evaluated on coronal reformatted images) and most frequent axial CT signs for diagnosing unilateral vocal cord palsy in this study.
| Correlations between | Spearman’s Rho (ρ) | p-value |
|---|
| Flattening of ipsilateral subglottic arch (evaluated on coronal reformatted images) and dilatation of ipsilateral pyriform sinus | 0.246* | 0.182** |
| Flattening of ipsilateral subglottic arch (evaluated on coronal reformatted images) and dilatation of ipsilateral laryngeal ventricle | 0.168* | 0.366** |
| Dilatation of ipsilateral laryngeal ventricle and dilatation of ipsilateral pyriform sinus | 0.800 (very strong) | 0.0001 (stastically significant) |
Correlation is significant at the 0.01 level (2-tailed). **Non significant
Discussion
The VCP is a common clinical problem which may reduce quality of life due to persistent hoarseness of voice and may be the first sign of a serious underlying pathology [1-3,5-6,8,14,22]. Any disease process along the course of vagus and/or RLN which interferes with normal functioning of nerve may result in VCP [2,13,15-16]. The most common causes include neoplasms, iatrogenic and trauma [7,11,18]. The frequent surgeries implicated in RLN palsy include surgeries related to thyroid, carotid artery and cervical spine [23]. Of tumours, lung, thyroid and aesophageal malignancies may result in unilateral VCP with bronchogenic malignancy being the most common cause [1,3,17]. Benign thyroid diseases may rarely be associated with RLN palsy [9,24].
Because of its longer mediastinal course, the left RLN is 1.4 to 2.5 fold more prone to injuries than the right [1,3-5,7,9,13-16]. We also found VCP on left side 2.4 times more frequently than the right (71% on left vs. 29% on right).
Isolated involvement of the RLN is more common than the vagal neuropathy which has reported incidence of nearly 10% [4,9,14]. In present study, 9.7% patients had imaging findings consistent with central vagal neuropathy which is in agreement with the previous studies [4,9,14]. Any lesion involving brainstem, base of skull, and carotid artery bifurcation may result in vagal neuropathy [9,14]. So, if proximal central neuropathy is suspected further evaluation of posterior fossa is warranted with Magnetic Resonance Imaging (MRI) [4,25] whereas CT is more sensitive for evaluation of infrahyoid neck and mediastinum [3,19,25].
Inspite of varied etiologies of VCP, in significant number of cases no offending lesion can be identified along the course of vagus/RLN and such cases are labeled as idiopathic [4,9,14,18]. In present study, despite of meticulous search, we failed to demonstrate any offending pathology in 64.5% cases which constituted idiopathic group. Our findings correlate with previous studies in which substantial numbers of patients with VCP were idiopathic [1,9].
Remaining 35.5% (11 out of 31) cases in present study were secondary to pathological involvement of RLN nerve by some morphological lesion. In one case a large aortic arch aneurysm was identified compressing the left RLN in AP window. Two cases of pancoast tumor were seen with infiltration into left RLN in superior mediastinum [Table/Fig-12]. Two cases each of hilar metastatic lymphnodes and hilar bronchogenic malignancy were seen with mediastinal invasion [Table/Fig-14]. One case of left upper lobe bronchogenic scar malignancy was also seen. Two malignant neck masses were identified, one was squamous cell carcinoma of right tonsil with metastatic cervical lymphadenopathy and other was conglomerated lymphnodal mass both invading into tracheo-oesophageal groove [Table/Fig-15]. One case of thyroid malignancy was seen with direct tumour infiltration into RLN. Tumours of lung origin were most frequent cause of secondary unilateral VCP in present study which is in accordance with previous studies [3,5,17].
a) Axial CECT section in 58 year old male with left VCP at the level of upper thorax show left hilar malignant mass with mediastinal invasion (*) b) Axial CECT section in same patient at the level of vocal cords shows typical sail sign (*) c) Coronal reformatted images in same patient show flattening of subglottic arch on left side (arrow) with dilatation of left laryngeal ventricle (*).
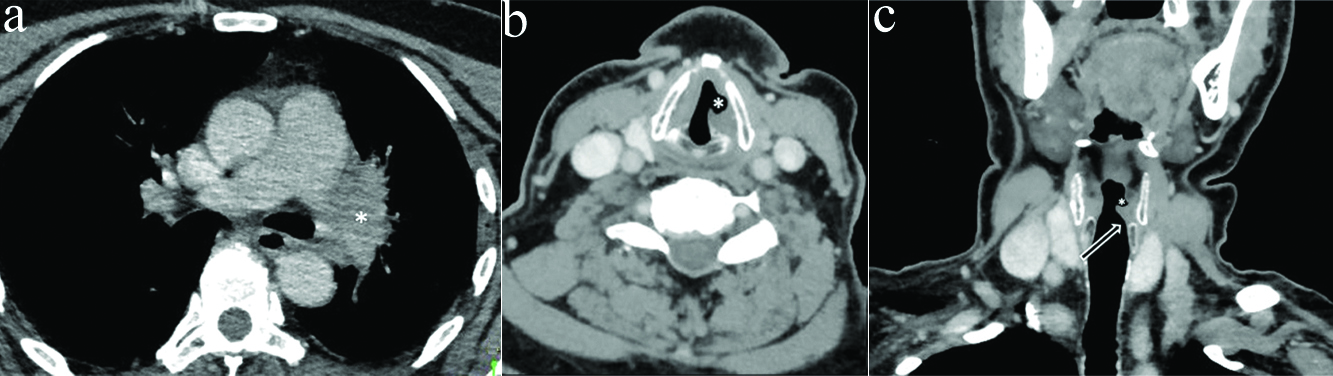
a) Axial CECT section in 70 year old male with left VCP at the level of hypopharynx show thickening and medialisation of left aryepiglottic fold (arrow) with dilatation of left pyriform sinus (*) b) Axial CECT section in same patient at lower level shows conglomerated left metastatic lymph nodal mass with invasion of tracheooesophageal groove (*) c) Coronal reformatted images in same patient show flattening of subglottic arch on left side (white arrow) with dilatation of left laryngeal ventricle (black arrow).
While most patients with VCP presents with hoarseness of voice, dysphonia, vocal fatigue and aspiration, a substantial number (~ 35%) may be asymptomatic. In such cases, VCP may be detected as an incidental finding and radiologist must be aware of the key imaging signs of VCP to alert the treating physician for further appropriate evaluation. The main purpose of imaging evaluation is to rule out any underlying treatable and potentially life threatening morphological condition. A CT study including section from skull base upto the carina is an ideal investigation of choice as it allows optimal evaluation of entire course of vagus/RLN and upper mediastinum [3,14,18-19].
Landman GH has described various (at least 10) findings of VCP on frontal contrast enhanced laryngoscopic and cine studies [6,9,14,21]. Most of these findings correlate well with the CT imaging except thinning of edge of paralysed cord, tilted interarytenoid notch and craniocaudal change in position of paralysed cord during inspiration and phonation [9].
Majority of the imaging signs associated with RLN are due to result of atrophy of posterior cricoarytenoid muscle which is the only abductor of vocal cord. The atrophy of this muscle combined with failure to abduct the cord results in anteromedial displacement of arytenoid, medial positioning of aryepiglottic fold and dilatation (passive) of pyriform sinus. The dilatation of laryngeal ventricle is also a passive result of ipsilateral cord atrophy. Sagging of paralysed cord results in ipsilateral subglottic fullness on axial sections [13-14,26].
In present study, we assessed the above imaging signs of VCP on MDCT and found that thickening and medial positioning of ipsilateral aryepiglottic fold was the only sign which was uniformly seen in all the cases of unilateral VCP. Two other frequent signs were dilatation of ipsilateral laryngeal ventricle (93.5%) and dilatation of ipsilateral pyriform sinus (87%) which showed very strong (Spearman’s Rho ρ-0.8000) and statistically significant correlation for unilateral VCP. The combination of any 2 out of these 3 signs on same side allowed us to achieve correct diagnosis of ipsilateral cord palsy in all the cases. Chin SC et al., detected all these three imaging findings in 77.5% of their cases while Garcia MM et al., and Limeme M et al., observed first two findings (thickening and medialisation of ipsilateral aryepiglottic fold and dilatation of ipsilateral laryngeal ventricle) in more than 75% of their cases [9,14,20]. Thus, the most common imaging signs of unilateral VCP in our study match with above studies with only difference in percentages. Higher prevalence of these signs in our study could be attributed to multidetector (128 slice) CT scanner used which enabled us to pick up even subtle findings in our cases.
The anteromedial displacement of ipsilateral arytenoids cartilage was observed in 58% cases in the present study which is some what comparable with study by Chin SC et al., who found this finding in 45% cases [9]. Another noteworthy finding is fullness of ipsilateral true cord. One might expect the asymmetric appearance of paralysed true cord to be very common imaging finding, however, fullness of paralysed cord was seen in only 35% of our cases which is in agreement with the previous study by Chin SC et al., [9]. The lower frequency of this finding may be explained due to possible difficulty in accurate alignment of the scanning plane with true cords.
Rest all the signs which we assessed were infrequently seen. Although, number of studies have evaluated various signs of VCP on CT but only a few of them have mentioned exact prevalence of these signs [4,6,9,13-14,20]. The relative percentages of these less commonly observed signs in present study more or less matched with the previous studies [9]. Thus, we believe that all these infrequent signs can merely act as minor supportive signs of VCP.
Two relatively newer signs of VCP on axial CT acquisition i.e., ‘sail sign’ and ‘mushroom sign’ have although been described previously, but to the best of our knowledge we could not found any literature regarding their exact prevalence in cases of unilateral VCP [4]. In the present study, sail sign was positive in 58% cases and mushroom sign in 48% cases. The sail sign was slightly more common than mushroom sign and both of these signs were seen in nearly half of our patients. So, we believe that these signs can be the potential supportive signs of unilateral VCP, and if present can aid in diagnosis.
Use of MDCT with three dimensional reconstructions has completely revolutionised the image analysis. However, only flattening of ipsilateral subglottic arch on coronal reformatted images have been found useful in evaluation of unilateral VCP [4,9,14]. The lower margin of true cord and lateral wall of subglottic larynx on coronal reformatted images normally form a superolateral concave contour during suspended respiration referred to as subglottic arch. This arch is lost or flattened on the side of VCP due to medially placed paralyzed true cord [4]. This finding was infrequently found in present study (positive in only 26% cases) and showed weak/very weak correlation with dilatation of ipsilateral pyriform sinus and ipsilateral laryngeal ventricle for diagnosing unilateral VCP [Table/Fig-16]. Chin SC et al., in their study found this finding on coronal reformatted images useful in cases with equivocal findings on axial sections [9]. Thus, stated that coronal CT images can be a useful adjunct in addition to axial images in difficult cases of VCP. However, they were not able to arrive at a reliable conclusion regarding routine use of these sections in all the patients and suggested further studies. Limeme M et al., also advocated use of coronal reformatted images in difficult cases to further refine the diagnosis [20]. On the other hand, Garcia MM et al., although, acknowledged enhanced capacity for laryngeal analysis with the advent of high resolution coronal reformatted images but still emphasised that axial sections allow correct diagnosis in majority of the patients [14].
a) Axial CECT section in 45 year old female with right VCP at the level of hypopharynx shows thickening and medialisation of right aryepigglotic fold (thick black arrow) with dilatation of right pyriform sinus (thin black arrow) b) Axial CECT section in same patient at the level of vocal cords shows typical sail sign (*) c) Coronal reformatted images showed dilated right lateral ventricle (thick white arrow) but maintained subglottic arch on right side (thin white arrow) inspite of right VCP.
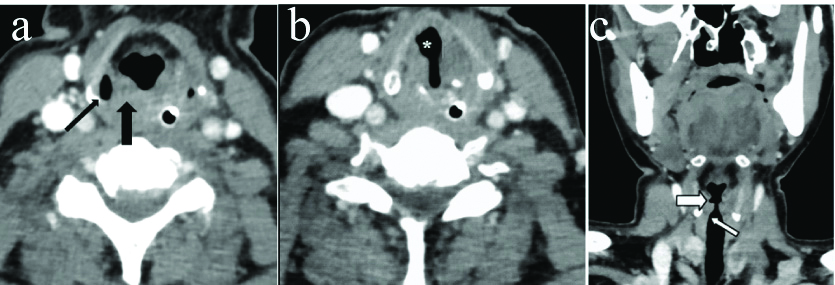
In the present study, four of our cases had findings on the opposite side which led to confusion in determining the exact side of VCP [Table/Fig-9,10,11 and 12]. All these cases had contralateral pyriform sinus dilatation with one of them also showing concurrent vallecular dilatation on opposite side. Strangely, flattening of ipsilateral subglottic arch on coronal images was not detected in any of these cases. So, we relied solely on axial imaging signs (thickening and medial positioning of ipsilateral aryepiglottic fold and ipsilateral dilatation of laryngeal ventricle) in these cases, which proved more than adequate to achieve correct diagnosis. Thus, we strongly feel that the routine axial CT acquisitions are sufficient for accurately diagnosing unilateral VCP even in cases with confusing contralateral findings.
Strength of study: Our study will boost the confidence of radiologists in diagnosing unilateral VCP on axial images alone, especially those who are still working in remote areas on lower end CT scanners where coronal reformatted images are not available most of the time.
Limitation
The present study had few limitations. Firstly, the results of the study cannot be applied to cases of bilateral VCP. Secondly, the present study was a single center study with a relatively small sample size, which might limit statistical relevance.
Conclusion
CT scan can reliably help in diagnosing VCP even in unsuspected and asymptomatic cases and can also help in detecting any clinically occult lesion along the course of vagus/RLN, thereby guiding timely and appropriate intervention. Thickening and medialisation of ipsilateral aryepiglottic fold is probably the single most reliable and consistent axial CT sign of unilateral VCP. Dilatation of ipsilateral laryngeal ventricle and ipsilateral pyriform sinus also correlate strongly with VCP on same side and act as major supportive signs of VCP. ‘Sail sign’ and ‘Mushroom sign’ are two relatively newer signs with potential to act as an additional supportive signs for unilateral VCP. Despite the increasing availability of MDCT, the coronal reformatted images may not offer any additional advantage in diagnosing unilateral VCP and axial sections still stay the gold standard to achieve an accurate diagnosis.
Correlation is significant at the 0.01 level (2-tailed). **Non significant