Introduction
Homocysteine is a thiol containing non essential amino acid formed from methionine by demethylation and is an intermediate product in One Carbon Metabolism (OCM). Deficiency of vitamin B12 and folic acid may cause serum Hcy concentration to rise. The role of folate in neurolation during early embryonic life has been well documented. Hcy delays the closure of the neural tube in chick embryo by inhibiting the transmethylation pathway [1]. It has been reported that patients with epilepsy experience Hyperhomocysteinaemia (hyper-Hcy) more frequently than general population mainly ascribable to the reduced activity of 5, 10-Methylene Tetrahydrofolate-Reductase (MTHFR) enzyme, deficiency of folate and vitamin B12 [2]. In addition, previous studies have demonstrated that hyper-Hcy is also induced by AEDs [3,4].
Out of four forms of Hcy that exist in plasma, about 1% exists as free thiol, 70-80% of Hcy is bound to plasma proteins and remaining 20-30% combines with itself or with other thiol including cysteine to form Hcy cysteine mixed disulfide [5]. The nutritional deficiency of either B12/folate or B6 is associated with increased levels of Hcy as the metabolism of Hcy requires them in transsulfuration or remethylation pathway [6].
The association of Hcy with various neurological disorders such as epilepsy, Parkinson’s disease, Alzheimer’s disease and multiple sclerosis has been established by several epidemiological and clinical studies as well [7]. However, the relation between Hcy and epilepsy remains controversial despite a growing evidence of the pro-convulsive effects of the increased Hcy level observed in animal studies [8]. Hcy and homocysteic acid brings about neuronal death by stimulating N-Methyl-D-Aspartate (NMDA) receptors and hence Hcy may reduce seizure threshold in epileptic patients [9].
Evidences show that Hcy can be produced in brain and transported from plasma into the brain and vice versa via bi-directional cellular transporters [10].
There is not much information available on the influence of AEDs on Hcy levels in epileptic patients. However, AED’s like carbamazepine, phenytoin and phenobarbital have been observed to cause folate, vitamin B6 and B12 deficiency [11]. A previous study has also suggested that enzyme inducers can directly modulate the activity of different liver enzymes which in turn may result in depletion of cofactors involved, leading to alteration in Hcy levels [12].
Brain inflammation occurs during epileptogenesis and contributes in determination of the seizure threshold in susceptible brain regions. Thus, it plays a role in seizure initiation and its recurrence [13]. In addition to inflammation, some of the pathways are up regulated immediately after epileptogenic injury and persists during latent phase which precedes spontaneous recurrent seizure. The brain inflammation has gained significance as a crucial contributor in the aetiopathogenesis of various Central Nervous System (CNS) disorders, including epilepsy as the auto inflammatory and autoimmune diseases are the pathological outcome of tissue inflammation [14]. CRP has emerged as an inflammatory marker and its role has been studied in non inflammatory neurological diseases; however, very few studies have been reported in epileptic seizures [15]. Hence, the present study was taken with an aim to find out the association of Hcy and hs-CRP with IGE. In addition, the levels have also been evaluated in association with the duration of AED therapy.
Materials and Methods
In this case control study, 100 diagnosed epileptic patients of IGE (as consulted by the statistician), (male:female=65:35) particularly idiopathic generalised tonic clonic seizures were recruited from Nizam’s Institute of Medical Sciences, Hyderabad from October 2012 to December 2014 and similarly, equal number of age and sex matched healthy controls were recruited from Institute of Genetics and Hospital for Genetic Diseases, Hyderabad with their written informed consent. Patients with trauma, major renal, hepatic, cardiac disease, cerebrovascular disease/infection, hypoxic-ischaemic encephalopathy, congenital CNS abnormalities, metabolic disorders, CNS malignant tumors and other degenerative disorders were excluded from the study. The study was approved by the Ethics Committee of the ‘Institute of Genetics and Hospital for Genetic Diseases’, as well as from the study hospital. The clinical diagnosis was made according to the admitted criteria by International League Against Epilepsy (ILAE) by an expert Epileptologist from Nizam’s Institute of Medical Sciences, Hyderabad. In addition, patients had normal intelligence (as evaluated by a psychologist) and the MRI brain was normal. The information on demographic features such as socioeconomic status, nutritional status and geographic area and seizure frequency, type of epilepsy as well as duration was collected in a specially designed proforma. The medical history such as drugs and duration of the therapy was also obtained about each patient. As IGE has an age related onset, we preferred 18 years as cut off age to differentiate “classical” and adult onset IGE on the basis of previous reports [16-18]. Further, the patients were divided into three subgroups viz., ≤1 year, 1-≤5 years and >5 years based on the duration of the AED treatment. Patients were classified into responders and nonresponders on the basis of their response to AEDs. Nonresponders included the patients who had one or more seizures in the previous six months despite being treated with at least two AED such as Phenytoin, Carbamazepine, Sodium Valproate, Lamotrigine etc.
A 3 mL of venous blood was collected from all subjects, in case of patients within 24 hours from the seizure attack, in sterile silicone tubes without addition of anticoagulants and centrifuged at 2,500 rpm for 20 minutes. The serum was collected in sterile tubes and was stored at -80°C until the levels of serum Hcy and hs-CRP were analysed. Serum Hcy levels were estimated by Centaur XP using ADVIA centaur Hcy and assay. Serum hs-CRP levels were assayed by ELISA method using commercially available kits procured from Diagnostics Biochem, Canada.
Statistical Analysis
The difference in the levels of Hcy and hs-CRP between patients and controls was evaluated by Student’s t-test, One-way analysis of variance (ANOVA) and the p-value of <0.05 was considered to be significant. Pearson correlation was used to evaluate the correlation between Hcy and hs-CRP.
Results
The mean age of IGE patients was 21.8±13.4 years where as in controls it was 23.4±15.3. The ratio between male and female was 65:35 and the family history of IGE was observed in 21% of patients. The mean age (in years) of the patients with IGE for age group <18 years and healthy controls was 7.6±1.96 and 10.5±1.85; and for patients in the age group >18 years and controls was 36.5±10.4 and 39.0±11.6 respectively. Out of 100 IGE patients, in majority (63%) the age of onset was <18 years and the remaining (37%) patients had the onset above the age of 18 years [Table/Fig-1].
Demographic profile of idiopathic generalised epilepsy patients and controls.
| Demographic features | Patients(n=100) | Controls(n=100) | p-value |
|---|
| Age (years) (4-69) (mean±SD) | 21.8±13.4 | 23.4±15.3 | 0.43 |
| <18 years (n=63)>18 years (n=37) | 7.6±1.9636.5±10.4 | 10.5±1.8539.0±11.6 | 0.000*0.26 |
| Gender (male: female) | 65:35 | 65:35 | |
| Family history (%) | 21% | 1% | |
Note: p<0.05* significant
The mean time since diagnosis was 14.8 years. The levels of Hcy and hs-CRP in patients belonging to different age groups with their respective controls have been presented in [Table/Fig-2]. The hs-CRP levels in both the patient groups (<18 years and > 18 years) were found to be significantly high as compared to their healthy controls (2.10±0.62, 0.56±0.28; 3.22±1.05, 0.48±0.20 mg/L respectively; p<0.05). Significantly elevated levels of serum Hcy were observed in patients of different age groups compared to their respective controls 14.6±1.8 μmol/L, 9.9±2.18 μmol/L (p<0.001) and 14.5±1.50 μmol/L, 9.33±1.79 μmol/L (p<0.05) respectively [Table/Fig-2].
Data showing the serum homocysteine and hs-CRP levels in patients and controls in different age groups.
| Variable | | Controls (mean±sd) | Patients (mean±sd) | p-value |
|---|
| Hcy (μmol/L) | Controls vs Patients (n=100) | 9.59±1.98 | 14.5±1.63 | <0.001* |
| <18 years age group (n=63) | 9.9±2.18 | 14.6±1.8 | <0.001* |
| >18 years age group (n=37) | 9.33±1.79 | 14.5±1.50 | <0.001* |
| hs-CRP (mg/L) | Controls vs Patients (n=100) | 0.53±0.25 | 2.52±0.97 | <0.001* |
| <18 years age group (n=63) | 0.56±0.28 | 2.10±0.62 | <0.001* |
| >18 years age group (n=37) | 0.48±0.20 | 3.22±1.05 | <0.001* |
Note: p<0.05 significant*
Hcy: Homocysteine, hs-CRP: High Sensitivity C-Reactive Protein
The serum levels of Hcy and hs-CRP in the patients of different epilepsy types have been given in [Table/Fig-3]. A significant difference in the levels of Hcy and hs-CRP was observed among different epilepsy types (p=0.007 and p<0.001 respectively). Serum levels of Hcy and hs-CRP in patient subgroups who were on AEDs treatment for different time periods (≤1 year, 1-≤5 years and >5 years) have been presented in [Table/Fig-4]. Significant difference in the levels of Hcy and hs-CRP was observed in the patient subgroups who were on AEDs for different time periods (p=0.003 and p=0.000 respectively). Significantly elevated levels of hs-CRP were noticed in non-responders compared to responders group (p<0.05) whereas, no significance was observed with Hcy levels [Table/Fig-4]. We also found that a positive correlation between the homocysteine, hs-CRP levels [Table/Fig-5,6] and the duration of the AED treatment. However, no significant difference in the levels of Hcy and hs-CRP was noted in the patients of either monotherapy (phenytoin, carbamazepine, sodium valproate) or polytherapy (p=0.99 and p=0.95 respectively) [Table/Fig-7].
The serum levels of hs-CRP and homocysteine in different epilepsy types.
| Parameter | Epilepsy types | p-value |
|---|
| Absence(n=15) | Myoclonic(n=35) | TC seizures only(n=50) |
|---|
| Hcy (μmol/L) | 14.05±1.16 | 14.4±1.48 | 15.05±1.03 | 0.007* |
| hs-CRP (mg/L) | 2.02±0.34 | 2.38±0.94 | 2.9±0.77 | 0.000* |
Note: p<0.05 significant*
Hcy: Homocysteine, hs-CRP: High Sensitivity C-Reactive Protein, TC: Tonic Clonic
Levels of homocysteine and hs-CRP in patients with different time duration of AEDs treatment and in responders vs non-responders.
| Variable | <1 year (mean±SD) (n=23) | 1-5 years (mean±SD) (n=32) | >5 years (mean±SD)(n=45) | p-value | Non-responders (n=33) | Responders (n=67) | p-value |
|---|
| Hcy (μmol/L) | 13.9±1.94 | 14.7±1.46 | 15.2±1.14 | 0.003* | 14.7±0.96 | 14.5±1.88 | 0.56 |
| hs-CRP (mg/L) | 1.92±0.54 | 2.10±0.59 | 3.12±1.02 | 0.000* | 3.38±0.99 | 2.09±0.61 | 0.000* |
Note: p<0.05 significant*
Hcy: Homocysteine, hs-CRP: High Sensitivity C-Reactive Protein
Correlation of homocysteine and hs-CRP in idiopathic generalised epilepsy patients.
| Variable | With variable | Sample size (n) | Sample correlation | Odds ratio (95% CI) | R2 | p-value |
|---|
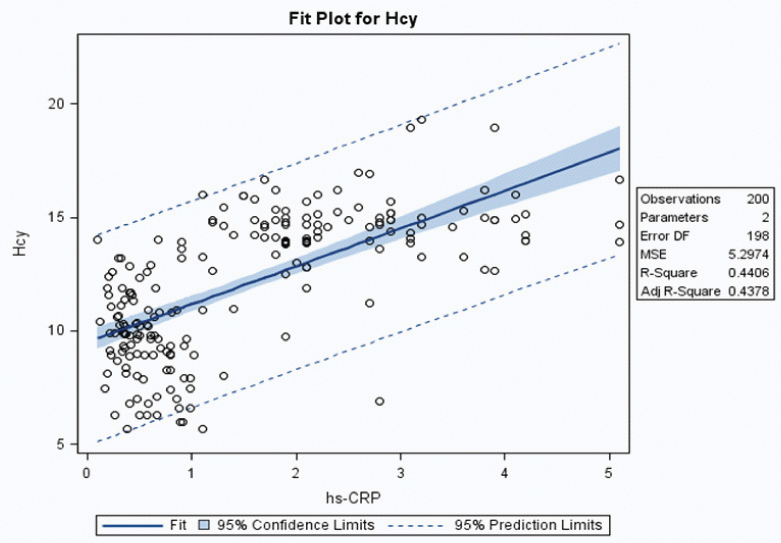
| Hcy | hs-CRP | 200 | 0.6638 | 0.6628 (0.57-0.73) | 0.44 | <0.0001* |
Note: p<0.05 significant*
Hcy: Homocysteine, hs-CRP: High Sensitivity C-Reactive Protein
Scatter plot with trendline showing correlation between homocysteine and high sensitivity C-reactive protein.
Comparison of results among idiopathic generalised epilepsy patients on monotherapy and polytherapy.
| Parameter | Monotherapy | Polytherapy (n=25) | p-value |
|---|
| Phenytoin (n=25) | Carbamazepine (n=25) | Valproate (n=25) |
|---|
| Hcy (μmol/L) | 14.5±1.6 | 14.56±1.62 | 14.62±1.59 | 14.6±1.58 | 0.99 |
| hs-CRP (mg/L) | 2.48±0.9 | 2.50±0.78 | 2.52±0.62 | 2.4±0.8 | 0.95 |
Note: p>0.05 not significant*
Hcy: Homocysteine, hs-CRP: High Sensitivity C-Reactive Protein
Discussion
It is well documented that Hcy levels are elevated both in brain as well as Cerebrospinal Fluid (CSF) of several neurological disorders [19]. Our study showed significantly elevated levels of Hcy in patients with IGE (p<0.05) and also in patients of different age groups (p<0.05) when compared to controls. In addition, a significant difference in levels of Hcy were noticed among the patient subgroups who were on AED treatment for different duration (p<0.05). Our results are in accordance with the previous studies in which elevated Hcy levels have been noticed in epileptic patients and also positively correlated with the duration of AED treatment [20,21]. The higher levels of Hcy in epileptic patients have been attributed to the AEDs inducing microsomal liver enzymes as well as increasing cytochrome P450 enzyme activities that may lead to a decrease in serum folate concentration resulting in Hcy accumulation [22].
It is known from previous studies that Hcy inhibits neurite outgrowth from primary cultured dopaminergic neurons and cerebellar purkinje neurons indicating that Hcy can inhibit communication between neurons [23]. Baldelli E et al., have reported that increased Hcy levels can enhance seizure activity and neurodegeneration [24].
Hyper-Hcy results in 10-40% of the epileptics particularly those on AEDs pharmacotherapy in both children and adults [25,26]. It has been observed that the patients on Enzyme-Inducing Anti Epileptic Drugs (EIAEDs) such as carbamazepine and valproic acid presented low levels of folate or B12 vitamin and higher levels of Hcy. However, supplementation with folate 5 mg/day or Vitamin B12 900 μg/day or with both, gained the normal levels of both vitamins and reduced plasma Hcy levels in three months [27]. In another study, a positive correlation was noticed between multidrug AED treatment, age and duration of treatment with elevated Hcy levels and low folate levels [24]. Similarly, Schwaninger M et al., and Ono H et al., also reported increased Hcy levels in the epileptic patients on polytherapy [28, 29]. In contrast to it, no correlation was found between the levels of Hcy, pyridoxal 5-phosphate, vitamin B12 and the duration of treatment [11]. Similarly, the results of monotherapy were also controversial. Some studies have reported the elevated Hcy levels in the patients on carbamazepine and valproic acid treatment [2,30,31]. Though, the mechanism by which AEDs bring about a decrease in serum folate is unknown but it has been postulated that AEDs may cause a decrease in intestinal absorption of folate or an induction of liver enzymes [32]. However, in the present study we did not find any significant difference of Hcy levels between the patients of mono and polytherapy.
Oxidative stress directly influences Cystathionine Beta Synthase (CBS) and methionine synthase causing disruption of redox homeostasis and contributes to neuronal damage [33]. Hcy also induces neurological dysfunction by means of oxidative stress and increases the neurotoxicity of amyloid beta (Aβ) by promoting oxidative stress [34].
Hyper-Hcy is an established risk factor for vascular diseases such as stroke, myocardial infarction and peripheral arterial disease [35]. Chuang YC et al., have reported that the long term use of older generation AEDs such as carbamazepine, phenotoin, valproate may contribute to the atherosclerosis in epileptic patients due to changes in the lipid profile and also due to the increased serum Hcy levels [36]. Major congenital malformations have also been noticed in children whose mothers receive AEDs. This has been attributed to teratogenic effects of high Hcy levels [37].
Several human and experimental studies have revealed that chronic inflammation may play a vital role in neurodegenerative processes. Proinflammatory cytokines are increased in rodent models of epilepsy and their activation has been well correlated with the frequency of seizures [38,39]. The contribution of specific inflammatory pathways to the mechanism of ictogenesis in epileptic tissue has been demonstrated in experimental models, but the exact contribution of these pathways in epileptogenesis is still under assessment. Elevated CRP levels have been reported in various diseases including atherosclerotic cardiovascular disease for which it is an important biomarker as well [40]. Higher levels of inflammatory markers such as CRP and Interleukin 6 (IL-6) have been observed in various neurological diseases [41]. Xu G et al., also claimed that elevated levels of CRP were detected in mild congnitive impairment patients and significantly higher levels of plasma IL-6 and CRP were noticed in nondemented children with Down’s Syndrome [42,43]. Increased levels of IL-6 and CRP have been demonstrated in patients with undiagnosed and untreated tonic clonic seizures without any demonstrable systemic or CNS infection [44]. In the present study, elevated hs-CRP levels were observed in IGE patients compared with healthy controls and our results were in accordance with the previous study where the higher concentrations of CRP have been noticed in epileptic patients and in paediatric epilepsy patients with frequent, refractory generalised motor seizures [15,45]. In another study, the base line concentrations of CRP were higher in patients with refractory epilepsy but no difference was found in comparison with healthy controls [15,46]. Significantly elevated hs-CRP levels were noticed in non-responders (refractory) when compared to responders (p<0.05). Further, the levels of hs-CRP positively correlated with the duration of the disease as well the duration of AED treatment (p<0.05). Our results are in accordance with a recent study in which elevated CRP levels have been demonstrated in paediatric epilepsy patients with frequent, refractory generalised motor seizures [15]. The elevated levels of hs-CRP in non-responders can be attributed to the contribution of inflammatory pathways in ictogenesis in epileptic tissue and thereby, causing neurodegeneration and intractable epilepsy.
CRP levels have also been associated with different epilepsy types. Patients with tonic clinic seizures showed higher levels of IL-6 compared with controls and also correlated with peripheral blood leucocyte count and CRP levels [44,47]. In the present study also, we found a significant difference in the hs-CRP concentrations between different epilepsy types particularly in the age group >18 years of tonic clonic seizures (p<0.05). Our data suggests that generalised tonic clonic seizures may stimulate CRP production. Similarly, Hcy levels were positively correlated with hs-CRP levels in IGE patients (p<0.05). It is assumed that inflammation is mediated by two different mechanisms i.e., through activation of nuclear factor-κβ or oxidative stress. The significant relation between Hcy and hs-CRP suggests that the association of Hcy with IGE may be dependent on inflammation related mechanisms.
Limitation
A more extensive study with a large sample size and for longer duration is required to attain a firm conclusion. We have not done the follow up study with individual AEDs which could be more informative.
Conclusion
Hyper-Hcy may be a known phenomenon in the epilepsy patients, who were on AEDs, either mono or polytherapy. It has been claimed that supplementation with cofactors of Hcy metabolism such as folic acid, Vitamin B12 and B6 can reduce the Hcy levels to normal in such patients. Since, chronic AED therapy can induce oxidative stress, we suggest that all the epileptic patients who are on AEDs whether mono or polytherapy should be screened for Hcy levels to avoid adverse effects of hyper-Hcy and be treated accordingly if the levels are found to be high. Hyper-Hcy can induce as well as promote oxidative stress and hence, it can be implicated in neurodegeneration in epilepsy. Elevated levels of hs-CRP in non-responders may be resulted by the contribution of inflammatory pathways in ictogenesis in epileptic tissue and thereby, causing neurodegeneration and intractable epilepsy.
Note: p<0.05* significant
Note: p<0.05 significant*
Hcy: Homocysteine, hs-CRP: High Sensitivity C-Reactive Protein
Note: p<0.05 significant*
Hcy: Homocysteine, hs-CRP: High Sensitivity C-Reactive Protein, TC: Tonic Clonic
Note: p<0.05 significant*
Hcy: Homocysteine, hs-CRP: High Sensitivity C-Reactive Protein
Note: p<0.05 significant*
Hcy: Homocysteine, hs-CRP: High Sensitivity C-Reactive Protein
Note: p>0.05 not significant*
Hcy: Homocysteine, hs-CRP: High Sensitivity C-Reactive Protein
[1]. Afman LA, Blom HJ, Drittij MJ, Brouns MR, van Straaten HW, Inhibition of transmethylation disturbs neurulation in chick embryosBrain Res Dev Brain Res 2005 158(1-2):59-65.10.1016/j.devbrainres.2005.06.00215996755 [Google Scholar] [CrossRef] [PubMed]
[2]. Karabiber H, Sonmezgoz E, Ozerol E, Yakinci C, Otlu B, Yologlu S, Effects of valproate and carbamazepine on serum levels of homocysteine, vitamin B12, and folic acidBrain Dev 2003 25(2):113-15.10.1016/S0387-7604(02)00163-8 [Google Scholar] [CrossRef]
[3]. Bochynska A, Lipczynska-Łojkowska W, Gugala-Iwaniuk M, Lechowicz W, Restel M, Graban A, The effect of vitamin B supplementation homocysteine metabolism and clinical state of patients with chronic epilepsy treated with carbamazepine and valproic acidSeizure 2012 21(4):276-81.10.1016/j.seizure.2012.01.01322360846 [Google Scholar] [CrossRef] [PubMed]
[4]. Coppola G, Ingrosso D, Operto FF, Signoriello G, Lattanzio F, Barone E, Role of folic acid depletion on homocysteine serum level in children and adolescents with epilepsy and different MTHFR C677T genotypesSeizure 2012 21(5):340-43.10.1016/j.seizure.2012.02.01122425007 [Google Scholar] [CrossRef] [PubMed]
[5]. Hankey GJ, Eikelboom JW, Homocysteine and vascular diseaseLancet 1999 354(9176):407-13.10.1016/S0140-6736(98)11058-9 [Google Scholar] [CrossRef]
[6]. Selhub J, Jacques PF, Wilson PW, Rush D, Rosenberg IH, Vitamin status and intake as primary determinants of homocysteinemia in an elderly populationJAMA 1993 270(22):2693-98.10.1001/jama.1993.035102200490338133587 [Google Scholar] [CrossRef] [PubMed]
[7]. Ientile R, Curro M, Ferlazzo N, Condello S, Caccamo D, Pisani F, Homocysteine, vitamin determinants and neuralogical diseaseFront Biosci. (Schol Ed) 2010 2:359-72.10.2741/s70 [Google Scholar] [CrossRef]
[8]. Kubova H, Folbergrova J, Mares P, Seizures induced by homocysteine in rats during ontogenesisEpilepsia 1995 36(8):750-56.10.1111/j.1528-1157.1995.tb01611.x7635093 [Google Scholar] [CrossRef] [PubMed]
[9]. Lipton SA, Kim WK, Choi YB, Kumar S, D’Emilia DM, Rayudu PV, Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptorProc Natl Acad Sci. USA 1997 94(11):5923-28.10.1073/pnas.94.11.59239159176 [Google Scholar] [CrossRef] [PubMed]
[10]. Remington G, Kapur S, Foussias G, Agid O, Mann S, Borlido C, Tetrabenazine augementation in treatment resistant schizophrenia: a 12-week, double-blind, placebo-controlled trialJ Clin Psychopharmacol 2012 32(1):95-99.10.1097/JCP.0b013e31823f913e22198452 [Google Scholar] [CrossRef] [PubMed]
[11]. Sener U, Zorlu Y, Karaguzel O, Ozdamar O, Coker I, Topbas M, Effects of common anti-epileptic drug monotherapy on serum levels of homocysteine, vitamin B12, folic acid and vitamin B6Seizure 2006 15(2):79-85.10.1016/j.seizure.2005.11.00216414291 [Google Scholar] [CrossRef] [PubMed]
[12]. Hamed SA, Nabeshima T, The high atherosclerotic risk among epileptics: the atheroprotective role of multivitaminsJ Pharm Sci 2005 98(4):340-53.10.1254/jphs.CRJ05003X [Google Scholar] [CrossRef]
[13]. Vezzani A, Iori V, Ravizza T, Bianchi ME, Maroso M, Activation of toll-like receptor 4 (TLR4) and receptor for advanced glycation end products (RAGE) signaling contributes to ictogenesis and epileptogenesisSociety for Neuroscience; Meeting, Washington DC Poster number 889.20 2011 [Google Scholar]
[14]. Vezzani A, Aronica E, Mazarati A, Pittman QJ, Epilepsy and brain inflammationExp Neurol 2013 244:11-21.10.1016/j.expneurol.2011.09.03321985866 [Google Scholar] [CrossRef] [PubMed]
[15]. Ishikawa N, Kobayashi Y, Fujii Y, Kobayashi M, Increased interleukin-6 and high-sensitivity C-reactive protein levels in pediatric epilepsy patients with frequent, refractory generalized motor seizuresSeizure 2015 25:136-40.10.1016/j.seizure.2014.10.00725455727 [Google Scholar] [CrossRef] [PubMed]
[16]. Janz D, Beck-Mannagetta G, Sander T, Do idiopathic generalised epilepsies share a common susceptibility gene? Neurology 1992 42(suppl 5):48-55. [Google Scholar]
[17]. Janz D, The idiopathic generalised epilepsies of adolescence with childhood and juvenile age of onsetEpilepsia 1997 38:04-11.10.1111/j.1528-1157.1997.tb01073.x9024180 [Google Scholar] [CrossRef] [PubMed]
[18]. Cutting S, Lauchheimer A, Barr W, Devinsky O, Adult-onset idiopathic epilepsy: clinical and behavioural featuresEpilepsia 2001 42(11):1395-98.10.1046/j.1528-1157.2001.14901.x11879340 [Google Scholar] [CrossRef] [PubMed]
[19]. Isobe C, Murata T, Sato C, Terayama Y, Increase of total homocysteine concentration in cerebrospinal fluid in patients with Alzheimer’s disease and Parkinson’s diseaseLife Sci 2005 77(15):1836-43.10.1016/j.lfs.2005.02.01415935398 [Google Scholar] [CrossRef] [PubMed]
[20]. Elliott JO, Jacobson MP, Haneef Z, Cardiovascular risk factors and homocysteine in epilepsyEpilepsy Res 2007 76(2-3):113-23.10.1016/j.eplepsyres.2007.07.00517714918 [Google Scholar] [CrossRef] [PubMed]
[21]. Eldeen ON, Abd Eldayem SM, Shatla RH, Omara NA, Elgammal SS, Homocysteine, folic acid and vitamin B12 levels in serum of epileptic childrenEgyptian Journal of Human Medical Genetics 2012 13(3):275-80.10.1016/j.ejmhg.2012.05.002 [Google Scholar] [CrossRef]
[22]. Moore JL, The significance of folic acid for epilepsy patientsEpilepsy and Behavior 2005 7(2):172-81.10.1016/j.yebeh.2005.05.02016054874 [Google Scholar] [CrossRef] [PubMed]
[23]. Heider I, Lehmensiek V, Lenk T, Müller T, Storch A, Dopaminergic neurotoxicity of homocysteine and its derivatives in primary mesencephalic culturesJ Neural Transm. Suppl 2004 (68):01-13.10.1007/978-3-7091-0579-5_1 [Google Scholar] [CrossRef]
[24]. Baldelli E, Leo G, Andreoli N, Fuxe K, Biagini G, Agnati LF, Homocysteine potentiates seizures and cell loss induced by pilocarpine treatmentNeuromolecular Med 2010 12(3):248-59.10.1007/s12017-009-8110-120033627 [Google Scholar] [CrossRef] [PubMed]
[25]. Huemer M, Ausserer B, Graninger G, Hubmann M, Huemer C, Schlachter K, Hyperhomocysteinemia in children treated with antiepileptic drugs is normalized by folic acid supplementationEpilepsia 2005 46(10):1677-83.10.1111/j.1528-1167.2005.00264.x16190942 [Google Scholar] [CrossRef] [PubMed]
[26]. Apeland T, Froyland ES, Kristensen O, Strandjord RE, Mansoor MA, Drug-induced perturbation of the aminothiol redox-status in patients with epilepsy: improvement by B-vitaminsEpilepsy Res 2008 82(1):01-06.10.1016/j.eplepsyres.2008.06.00318644700 [Google Scholar] [CrossRef] [PubMed]
[27]. Linnebank M, Moskau S, Semmler A, Widman G, Stoffel-Wagner B, Weller M, Antiepileptic drugs interact with folate and vitamin B12 serum levelsAnn Neurol 2011 69(2):352-59.10.1002/ana.2222921246600 [Google Scholar] [CrossRef] [PubMed]
[28]. Schwaninger M, Ringleb P, Winter R, Kohl B, Fiehn W, Rieser PA, Elevated plasma concentrations of homocysteine in antiepileptic drug treatmentEpilepsia 1999 40(3):345-50.10.1111/j.1528-1157.1999.tb00716.x10080517 [Google Scholar] [CrossRef] [PubMed]
[29]. Ono H, Sakamoto A, Mizoguchi N, Sakura N, The C677T mutation in the methylenetetrahydrofolate reductase gene contributes to hyperhomocysteinemia in patients taking anticonvulsantsBrain Dev 2002 24(4):223-26.10.1016/S0387-7604(02)00004-9 [Google Scholar] [CrossRef]
[30]. Apeland T, Mansoor MA, Strandjord RE, Vefring H, Kristensen O, Folate, homocysteine and methionine loading in patients on carbamazepineActa Neurol Scand 2001 103(5):294-99.10.1034/j.1600-0404.2001.103005294.x11328204 [Google Scholar] [CrossRef] [PubMed]
[31]. Vilaseca MA, Monaos E, Arthuch R, Colome C, Farre Valls C, Anti-epileptic drug treatment in children: hyperhomocysteinemia, B-vitamins and the 677C ?T mutation of the methylenetetrahydrofolate reductase geneEur J Paediatr Neurol 2000 4(6):269-77.10.1053/ejpn.2000.037911277368 [Google Scholar] [CrossRef] [PubMed]
[32]. Siniscalchi A, Mancuso F, Gallelli L, Ferreri Ibbadu G, Mercuri NB, De Sarro G, Increase in plasma homocysteine levels induced by drug treatments in neurologic patientsPharmacol Res 2005 52(5):367-75.10.1016/j.phrs.2005.05.01316039138 [Google Scholar] [CrossRef] [PubMed]
[33]. Vitvitsky V, Dayal S, Stabler S, Zhou Y, Wang H, Lentz SR, Perturbations in homocysteinelinked redox homeostasis in a murine model for hyperhomocysteinemiaAm J Physiol Regul Integr Comp Physiol 2004 287(1):R39-46.10.1152/ajpregu.00036.200415016621 [Google Scholar] [CrossRef] [PubMed]
[34]. James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autismAm J Clin Nutr 2004 80(6):1611-17.10.1093/ajcn/80.6.161115585776 [Google Scholar] [CrossRef] [PubMed]
[35]. Graham IM, Daly LE, Refsum HM, Robinson K, Brattstrom LE, Ueland PM, Plasma homocysteine as a risk factor for vascular disease. The European concerted action projectJAMA 1997 277(22):1775-81.10.1001/jama.1997.035404600390309178790 [Google Scholar] [CrossRef] [PubMed]
[36]. Chuang YC, Chuang HY, Lin TK, Chang CC, Lu CH, Chang WN, Effects of long-term antiepileptic drug monotherapy on vascular risk factors and atherosclerosisEpilepsia 2012 53(1):120-28.10.1111/j.1528-1167.2011.03316.x22085257 [Google Scholar] [CrossRef] [PubMed]
[37]. Hernandez-Diaz S, Smith CR, Shen A, Mittendorf R, Hauser WA, Yerby M, Comparative safety of antiepileptic drugs during pregnancyNeurology 2012 78(21):1692-99.10.1212/WNL.0b013e3182574f3922551726 [Google Scholar] [CrossRef] [PubMed]
[38]. Ravizza T, Gagliardi B, Noe F, Boer K, Aronica E, Vezzani A, Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsyNeurobiol Dis 2008 29(1):142-60.10.1016/j.nbd.2007.08.01217931873 [Google Scholar] [CrossRef] [PubMed]
[39]. Rijkers K, Majoie HJ, Hoogland G, Kenis G, De Baets M, Vles JS, The role of interleukin-1 in seizures and epilepsy: A critical reviewExp Neurol 2009 216(2):258-71.10.1016/j.expneurol.2008.12.01419162013 [Google Scholar] [CrossRef] [PubMed]
[40]. Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Chambless LE, Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident ischemic stroke in middle-aged men and women in the atherosclerosis risk in communities (ARIC) studyArch Intern Med 2005 165(21):2479-84.10.1001/archinte.165.21.247916314544 [Google Scholar] [CrossRef] [PubMed]
[41]. Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Inflammatory proteins in plasma and the risk of dementia: the Rotterdam studyArch Neurol 2004 61(5):668-72.10.1001/archneur.61.5.66815148142 [Google Scholar] [CrossRef] [PubMed]
[42]. Xu G, Zhou Z, Zhu W, Fan X, Liu X, Plasma C-reactive protein is related to cognitive deterioration and dementia in patients with mild cognitive impairmentJ Neurol Sci 2009 284(1-2):77-80.10.1016/j.jns.2009.04.01819419739 [Google Scholar] [CrossRef] [PubMed]
[43]. Licastro F, Chiappelli M, Ruscica M, Carnelli V, Corsi MM, Altered cytokine and acute phase response protein levels in the blood of children with downs syndrome: relationship with dementia of Alzheimer’s typeInt J Immunopathol Pharmacol 2005 18(1):165-72.10.1177/03946320050180011715698521 [Google Scholar] [CrossRef] [PubMed]
[44]. Peltola J, Laaksonen J, Haapala AM, Hurme M, Rainesalo S, Keranen T, Indicators of inflammation after recent tonic-clonic epileptic seizures correlate with plasma interleukin-6 levelsSeizure 2002 11(1):44-46.10.1053/seiz.2001.057511888259 [Google Scholar] [CrossRef] [PubMed]
[45]. Tan TY, Lu CH, Chuang HY, Lin TK, Liou CW, Chang WN, Long-term antiepileptic drug therapy contributes to the acceleration of atherosclerosisEpilepsia 2009 50(6):1579-86.10.1111/j.1528-1167.2009.02024.x19292757 [Google Scholar] [CrossRef] [PubMed]
[46]. Mintzer S, Skidmore CT, Abidin CJ, Morales MC, Chervoneva I, Capuzzi DM, Effects of antiepileptic drugs on lipids, homocysteine, and C-reactive proteinAnn Neurol 2009 65(4):448-56.10.1002/ana.2161519296463 [Google Scholar] [CrossRef] [PubMed]
[47]. Alapirtti T, Waris M, Fallah M, Soilu-Hanninen M, Makinen R, Kharazmi E, C-reactive protein and seizures in focal epilepsy: a video-electroencephalographic studyEpilepsia 2012 53(5):790-96.10.1111/j.1528-1167.2012.03449.x22462619 [Google Scholar] [CrossRef] [PubMed]