Amyloidosis is a protein deposition disorder caused by pathologic accumulation of fibrils, leading to organ dysfunction. The newest protein is Leukocyte Cell-derived Chemotaxin 2 Amyloidosis (ALECT2), which shows ethnic predilection for Hispanics, Middle Eastern, and other minority groups. We report a case of 71-year-old Persian male with history of hepatitis B and resected hepatocellular carcinoma who presented with acute onset jaundice and abnormal liver function tests. Liver biopsy performed for diagnostic workup revealed hepatic ALECT2 with distinct signet-ring like globular proteinaceous deposits infiltrating hepatic parenchyma, mimicking epithelioid/histiocytic neoplasm. The infiltrative spherular material was positive for Congo red and negative for pan-keratin stains. Amyloid protein analysis by Liquid Chromatography tandem Mass Spectrometry (LC MS/MS) identified a peptide profile indicative of ALECT2. Although, ALECT2 accounts for the second most common cause of hepatic amyloidosis, only limited cases of hepatic ALECT2 are reported in the literature and little is known about patient management, especially the role of transplant as curative option. In summary, hepatic ALECT2 is an emerging disorder with relative high prevalence that deserves morphologic recognition. The report intends to emphasise distinctive morphology for accurate diagnosis and understanding its pathogenesis, clinical significance, and therapeutic strategies.
Case Report
A 68-year-old (age at the initial presentation) Persian male with history of resolved hepatitis B a year ago and a solitary hepatic lesion incidentally discovered six months ago was transferred from an outside institution with acute onset jaundice and elevated liver enzyme levels, as per medical note accompanied with the patient. After a careful review of the Magnetic Resonance Imaging (MRI) done at previous centre, the lesion was suspected to be malignant and the patient underwent resection of the left lateral segment of the liver to remove the solitary lesion. Histopathology demonstrated hepatocellular carcinoma, Grade 3-4, with microscopic lymph-vascular invasion. At that time, his Model for End-stage Liver Disease (MELD) score was 11 and was referred to a transplant office for an evaluation for possible transplant.
The transplant laboratory evaluation results were as follows: HbSAg negative, HBsAb positive, HbcAb positive, HBV DNA <20, HCV Ab negative, platelets 101×103/uL, Aspartate Transaminase (AST) 39 U/L, Alanine Transaminase (ALT) 20 U/L, alkaline phosphatase 139 U/L, total bilirubin 1.8 mg/dL, creatinine 0.85 mg/dL, Albumin 3.4 gm/dL, total protein 6.5 gm/dL, INR 1.2, ceruloplasmin 16.6 mg/dL, A1AT phenotype MM. Possible underlying autoimmune disease was excluded with negative laboratory results of antibodies, including anti-nuclear, anti-double stranded, anti-SSA, anti-SSB, anti-smith, anti-ribonucleoprotein, anti-centromere, anti-scl-70.
Despite surgical resection, the patient’s hepatic condition continued to worsen and showed signs of portal hypertension with variceal haemorrhage and pedal oedema. He remained relatively stable for approximately three years, but at the age of 71, he presented to the emergency department again with jaundice. Laboratory result demonstrated the following findings: direct bilirubin 34.9 mg/dL, total bilirubin 52.2 mg/dL, AST 106 U/L, ALT 67 U/L, INR 2.4, creatinine 2.56 mg/dL, platelets 101×103/μL. Magnetic Resonance Cholangiopancreatography (MRCP) showed no identifiable lesions, excluding recurrence of hepatocellular carcinoma.
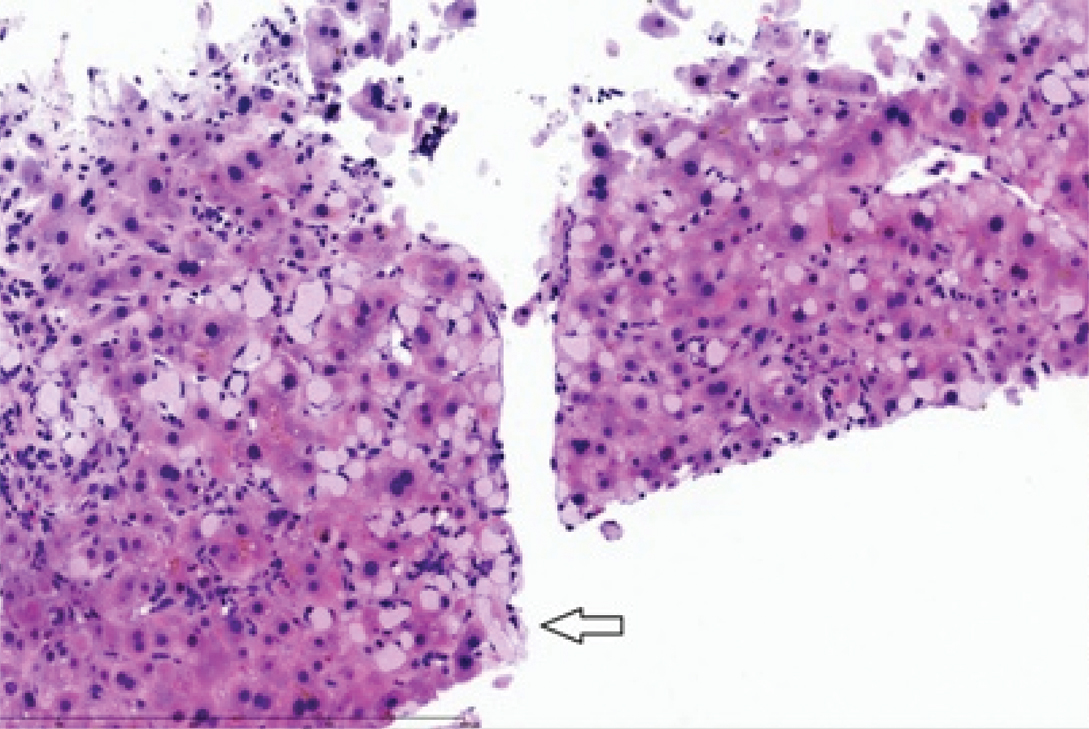
The patient underwent transjugular liver biopsy and multiple core biopsies of green-brown liver core tissues were submitted. The liver biopsy showed chronic hepatitis with prominent cholestasis and bridging fibrosis consistent with cirrhosis (Stage 4). There was no evidence of hepatocellular carcinoma in the submitted sample. However, multifocal prominent amorphous spherular eosinophilic material coursing through the hepatic parenchyma, featuring signet-ring like epithelioid morphology and mimicking signet-ring cell carcinoma was noted [Table/Fig-1a,b].
Histology section of liver biopsy showing spherular material with signet-ring features infiltrating hepatic parenchyma (arrow) (H&E, 20X).
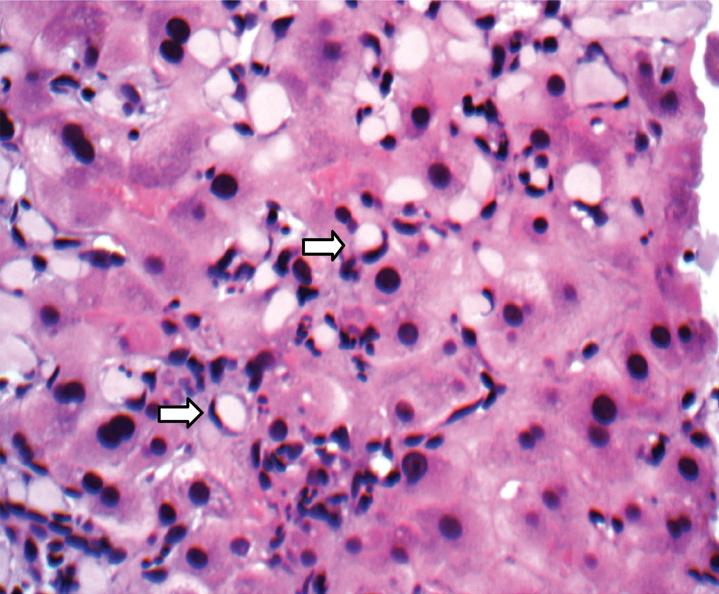
Signet-ring like morphology at higher magnification (arrow) (H&E, 40X).
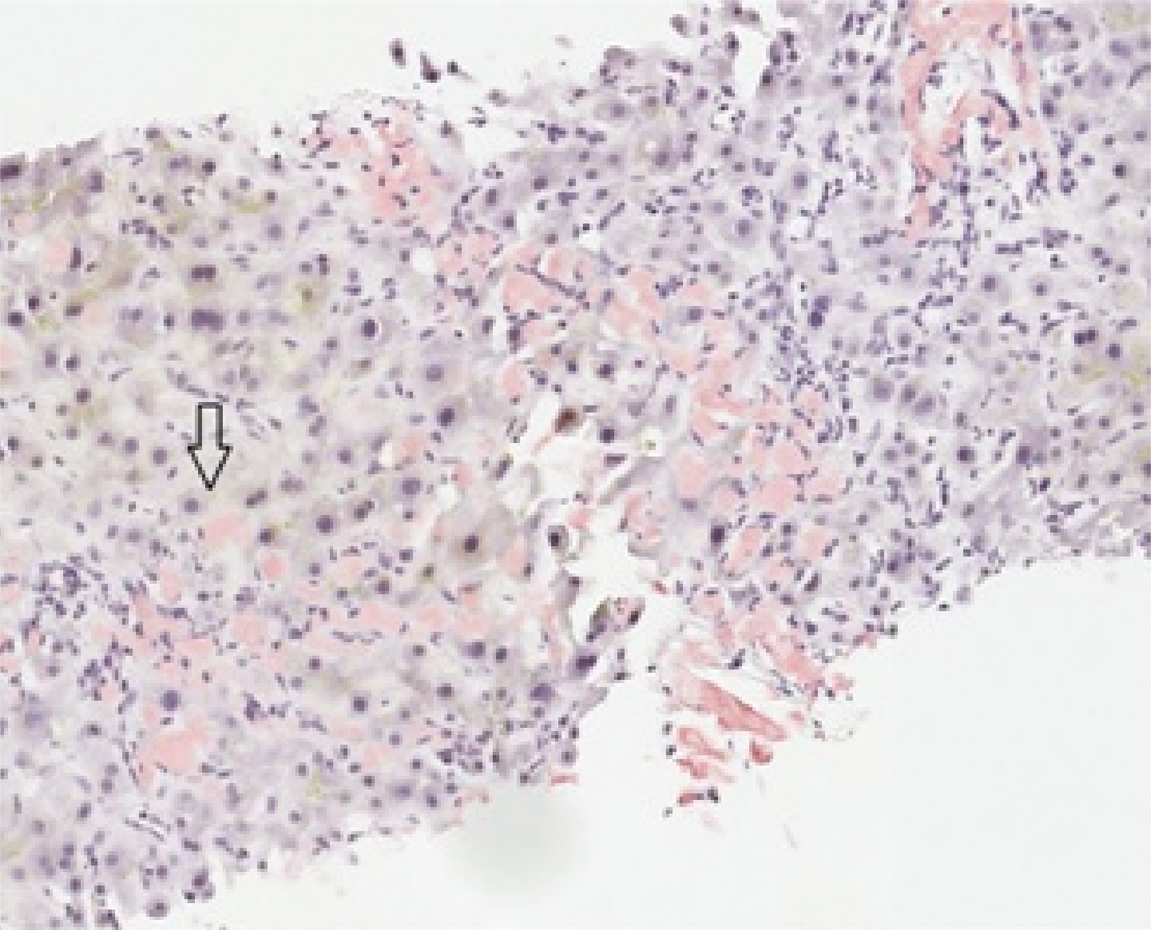
Immunohistochemistry stains for pan-keratin and CD68 were negative in the infiltrating spherular deposits, excluding epithelial or histiocytic lesions. Congo red stain demonstrated congophilic lesions [Table/Fig-1c] and was positive for apple green birefringence, confirming hepatic amyloidosis. Amyloid protein analysis was performed using LC MS/MS and detected a peptide profile consistent with Leukocyte Cell-derived Chemotaxin-2 (LECT2) type amyloid. From the time of admission, the patient continued to decompensate, developing acute kidney failure, liver failure, altered mental status, and disseminated intravascular coagulation. Despite the resuscitative effort and symptomatic treatment, the patient expired one month after the admission date.
Strongly congophilic amyloid coursing through hepatic parenchyma (arrow) (Congo red stain, 20X).
Discussion
Amyloidosis is a protein deposition disorder caused by pathologic accumulation of amyloid fibrils leading to tissue damage [1]. Amyloid represents a group of proteins featuring a beta-pleated structure and appears amorphous under light microscopy and fibrillar under electronic microscopy. The Congo red dye stains all β-pleated proteins and shows apple green birefringence under polarised light, establishing a simple diagnosis of amyloidosis [1]. Currently, over 30 types of proteins have shown to form amyloid deposits in humans [1]. ALECT2 is the newest member of recognised amyloid described by Benson MD et al., following isolation of protein from a patient with clear cell renal carcinoma [2]. Mereuta OM et al., demonstrated that ALECT2 was the second most common cause of hepatic amyloidosis, followed by Light chain Amyloidosis (AL) type [3], while Larsen CP et al., conducted largest autopsy series in 2016 to observe ethnic predilection of ALECT2 for Hispanics [4]. In the same year, Dogan A reported in his review paper that ALECT2 had been described in Punjabis, Egyptians, Arabs, First Nations People in Canada, and Native Americans [5]. The ethnic distribution combined with lack of diagnostic tools may have attributed to the delayed and under-recognition of ALECT2 [5] in the past.
Although, ALECT2 is more frequently described in the kidney, it is mainly produced by hepatocytes and functions as neutrophil chemotatic factor and growth stimulator of chondrocytes and osteoblasts [6]. LECT2 gene was detected on chromosome 5q31.1 by fluorescence in situ hybridisation located in close proximity to cytokine gene [7]. A genetic analysis of ALECT2 protein characterised the protein as wild-type with a G/A polymorphism in nucleotide 172 in exon 3, which was a speculation for pathogenesis [8]. However, this polymorphic site was thought to be common and was unlikely to be the primary cause of ALECT2 amyloidosis. Instead, experts theorised that upregulation of LECT2 gene expression either constitutively or secondary to hepatocyte damage might have resulted in amyloid genesis [3]. A recent case report described cytogenetic abnormality of der(7)add(7)(q34) pattern in a patient with renal ALECT2 [9]. With greater recognition and identification of ALECT2, a larger genetic study can establish undetermined genetic association or discover environmental factors.
Traditionally, the two widely accepted histologic patterns of hepatic amyloidosis include a sinusoidal linear pattern and a vascular linear pattern. Recently, a globular pattern of eosinophilic hepatic amyloid deposits along the sinusoidal spaces, periportal parenchyma, portal triad, and around venules was described [3,10,11]. Chandan VS et al., observed that all of 27 cases of hepatic ALECT2 showed a globular pattern of portal and/or sinusoidal deposition, concluding that this pattern is highly specific and distinctive for the ALECT2 subtype [10]. Similarly, our case featured a globular morphology that involved the sinusoids, consistent with previous findings. However, the unfamiliarity with the distinctive globular pattern simulated signet-ring like structures infiltrating through hepatic parenchyma, raising consideration of wide differentials from histiocytic lesions to signet-ring type carcinoma, which was excluded by negative immunohistochemistry stains for CD68 and pan-keratin. This case report emphasises the distinctive morphology of ALECT2 and helps in the increased awareness and accurate recognition of this subtype of amyloid.
Clinical management is determined by the type of amyloidosis, therefore accurate recognition and ancillary testing for identification is necessary. Commercially available immunohistochemistry analysis is a common method for typing, but high background staining and lack of specific antibody reagents to detect all 30 different proteins is a challenge [5]. Paueksakon P et al., reported that LECT2 stain produced high false positive rate and suggested LC MS/MS for accurancy [12]. On the other hand, Larsen CP et al., and Chandan VS et al., successfully identified LECT2 with high sensitivity and specificity using immunohistochemistry analysis [4,10]. Larsen C stated that aggressive high antigen retrieval method can result in high false positive rate, possibly explaining the discrepancy in results from Paueksakon’s study [13]. Alternatively, laser microdissection and MS-based proteomic analysis offer greater sensitivity and specificity by assessing the chemical composition [14]. Due to clinical implications, the aforementioned ancillary testing should be considered in all amyloidosis for accurate identification of the subtype and appropriate management of these patients.
Previously, ALECT2 was thought to deposit in various organ systems without causing damage, as the majority of patients were incidentally discovered while evaluating for other liver disorders [7]. However in our case, the patient showed signs and symptoms of portal hypertension and progressed to cirrhosis despite complete resolution of hepatitis B virus and resection of hepatocellular carcinoma. The patient’s unfortunate final outcome demonstrates the need of discussing and understanding this entity in a greater detail. The possible role of ALECT2 in liver damage was further supported by a similar case report that described a patient suffering from portal hypertension in presence of hepatic ALECT2 [11]. An in vitro study conducted by Ong HT et al., demonstrated that LECT2 expression was significantly reduced in hepatocellular carcinoma and overexpression inhibited tumor growth [15]. It is interesting to note that, our patient presented with concomitant HCC and amyloidosis. The oncologic implication of LECT2 requires further confirmatory in vivo studies.
Little is known about clinical management of hepatic ALECT2. Unlike AL type that requires assessment of plasma cell dyscrasia, pathogenesis of ALECT2 does not support the indication for bone marrow biopsy. For those who may present with concomitant monoclonal gammopathy, it is essential to distinguish AL type from ALECT2 to avoid misdiagnosis and unnecessary treatment with aggressive chemotherapy or stem cell transplant. Due to the patient’s liver condition, the possibility of a liver transplant was clinically considered. Unlike transthyretin type that demonstrated good prognosis with liver transplant [16], there are no known cases of liver transplant in patients with ALECT2 in the literature, precluding determination of its curative potential. The authors speculate that recurrence is a theoretical concern in transplanted organ since LECT2 is a wild-type protein that may accumulate secondary to over-expression of LECT2 gene or hepatocyte damage [3].
Conclusion
ALECT2 is a recently identified emerging disorder with relative high prevalence in specific patient populations that deserves increased awareness. Although hepatic involvement is frequent with systemic amyloidosis, literature on distinctive morphology of hepatic ALECT2 is limited. This case report emphasises the specificity of previously described globular morphology of ALECT2 in increasing diagnostic accuracy and navigating the potential challenges of clinical management.
Disclosure
Abstract was accepted for presentation at the annual meeting of the College of American Pathologists, October 8-11, 2017, Gaylord National, Maryland.
[1]. Sipe JD, Benson MD, Buxbaum JN, Ikeda SI, Merlini G, Saraiva MJ, Nomenclature 2014: amyloid fibril proteins and clinical classification of the amyloidosisAmyloid 2014 21(4):221-24.10.3109/13506129.2014.96485825263598 [Google Scholar] [CrossRef] [PubMed]
[2]. Benson MD, James S, Scott K, Liepnieks JJ, Kluve-Beckerman B, Leukocyte chemotactic factor 2: a novel renal amyloid proteinKidney Int 2008 74(2):218-22.10.1038/ki.2008.15218449172 [Google Scholar] [CrossRef] [PubMed]
[3]. Mereuta OM, Theis JD, Vrana JA, Law ME, Grogg KL, Dasari S, Leukocyte cell-derived chemotaxin 2 (LECT2)–associated amyloidosis is a frequent cause of hepatic amyloidosis in the United StatesBlood 2014 123(10):1479-82.10.1182/blood-2013-07-51793824415538 [Google Scholar] [CrossRef] [PubMed]
[4]. Larsen CP, Beggs ML, Wilson JD, Lathrop SL, Prevalence and organ distribution of leukocyte chemotactic factor 2 amyloidosis (ALECT2) among decedents in New MexicoAmyloid 2016 23(2):119-23.10.3109/13506129.2016.114511026912093 [Google Scholar] [CrossRef] [PubMed]
[5]. Dogan A, Amyloidosis: Insights from ProteomicsAnnu Rev Pathol: Mechanisms of Disease 2016 12:277-304.10.1146/annurev-pathol-052016-10020027959636 [Google Scholar] [CrossRef] [PubMed]
[6]. Picken MM, Alect2 amyloidosis: primum non nocere (first, do no harm)Kidney Int 2014 86(2):229-32.10.1038/ki.2014.4525079018 [Google Scholar] [CrossRef] [PubMed]
[7]. Yamagoe S, Kameoka Y, Hashimoto K, Mizuno S, Suzuki K, Molecular cloning, structural characterization, and chromosomal mapping of the human LECT2 geneGenomics 1998 48(3):324-29.10.1006/geno.1997.51989545637 [Google Scholar] [CrossRef] [PubMed]
[8]. Murphy CL, Wang S, Kestler D, Larsen C, Benson D, Weiss DT, Leukocyte chemotactic factor 2 (LECT2)-associated renal amyloidosis: a case seriesAm J Kidney Dis 2010 56(6):1100-07.10.1053/j.ajkd.2010.08.01320951486 [Google Scholar] [CrossRef] [PubMed]
[9]. Chakrabarti A, Samal P, Chakrabartty J, Amyloidosis: The newer discovered ALECT2 associated with der7q add (7)J Clin Diagn Res 2016 10(9):ED04-05.10.7860/JCDR/2016/21705.855527790444 [Google Scholar] [CrossRef] [PubMed]
[10]. Chandan VS, Shah SS, Lam-Himlin DM, Petris GD, Mereuta OM, Dogan A, Globular hepatic amyloid is highly sensitive and specific for LECT2 amyloidosisAm J Surg Pathol 2015 39(4):558-64.10.1097/PAS.000000000000037325602789 [Google Scholar] [CrossRef] [PubMed]
[11]. Damlaj M, Amre R, Wong P, How J, Hepatic ALECT-2 Amyloidosis causing portal hypertension and recurrent variceal bleedingAm J Clin Pathol 2014 141(2):288-91.10.1309/AJCPCLK54RKXTRDI24436280 [Google Scholar] [CrossRef] [PubMed]
[12]. Paueksakon P, Fogo AB, Sethi S, Leukocyte chemotactic factor 2 amyloidosis cannot be reliably diagnosed by immunohistochemical stainingHum Pathol 2014 45:1445-50.10.1016/j.humpath.2014.02.02024792621 [Google Scholar] [CrossRef] [PubMed]
[13]. Larsen C, Leukocyte chemotactic factor 2 amyloidosis can be reliably diagnosed by immunohistochemical stainingHum Pathol 2014 45(10):217910.1016/j.humpath.2014.06.02425123072 [Google Scholar] [CrossRef] [PubMed]
[14]. Gilbertson JA, Theis JD, Vrana JA, Lachmann H, Wechalekar A, Whelan C, A comparison of immunohistochemistry and mass spectrometry for determining the amyloid fibril protein from formalin-fixed biopsy tissueJ Clin Pathol 2015 68(4):314-17.10.1136/jclinpath-2014-20272225637636 [Google Scholar] [CrossRef] [PubMed]
[15]. Ong HT, Tan PK, Wang SM, Hian Low DT, Ooi LL, Hui KM, The tumor suppressor function of LECT2 in human hepatocellular carcinoma makes it a potential therapeutic targetCancer Gene Ther 2011 18:399-406.10.1038/cgt.2011.521394108 [Google Scholar] [CrossRef] [PubMed]
[16]. Ericzon BG, Wilczek HE, Larsson M, Wijayatunga P, Stangou A, Pena JR, Liver transplantation for hereditary transthyretin amyloidosis: after 20 years still the best therapeutic alternative?Transplantation 2015 99(9):1847-54.10.1097/TP.000000000000057426308415 [Google Scholar] [CrossRef] [PubMed]