Plasmablastic Lymphoma (PBL) is a rare and aggressive form of Non-Hodgkin Lymphoma (NHL), initially described in the oral cavity of Human Immunodeficiency Virus (HIV) positive individuals. Recently, it has been described in HIV negative patients and at several extraoral locations. It shows heterogeneous cytological findings but has a distinct immunophenotype. Knowledge of the cytomorphologic spectrum of PBL and detection of plasma cell markers by ancillary techniques help in achieving the correct diagnosis. Here, we present a 15-year-old HIV positive boy who presented with left periorbital, left chest wall and perianal swellings. A diagnosis of PBL was offered on Fine Needle Aspiration Cytology (FNAC) in conjunction with immunocytochemistry and was confirmed on histopathology with immunohistochemistry.
Cytology, Fine needle aspiration, Human immunodeficiency virus, Non-hodgkin lymphoma
Case Report
A 15-year-old boy presented with protrusion of the left eye, left anterior chest wall and perianal swellings for one month [Table/Fig-1]. He belonged to low socio economic group and a known HIV positive on anti-retroviral therapy for two years. On examination, the anterior chest wall swelling was soft to firm, and measured 4x3 cm. The perianal swelling was 2x1 cm, soft and non tender. Ophthalmic examination showed left sided proptosis, exposure keratopathy and perception of light only. Ultrasonography of the chest wall swelling showed a well defined heterogenous lesion, with a differential diagnosis of an abscess, or haematoma. Computed Tomography scan of head and neck however, suggested a multicompartmental aggressive neoplastic lesion involving pre and post septal compartments of the left orbit, left and right maxillary sinuses, half of middle cranial fossa and left buccal space with severe erosion of bones.
Clinical photograph: Left orbital and anterior chest wall swellings.
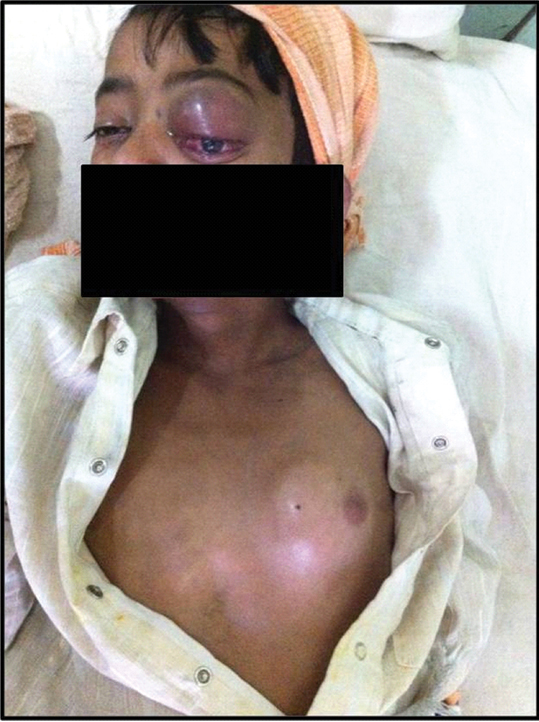
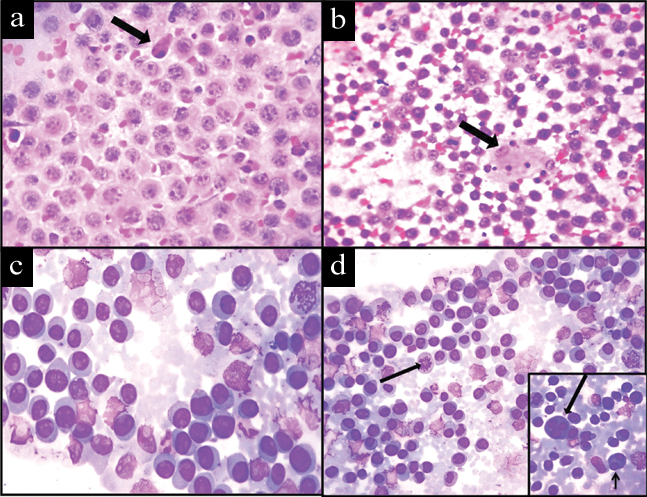
His complete blood count was within normal limits and CD4 count was 33/μL. His serum protein electrophoresis was within normal limits and urine for Bence-Jones protein was negative. FNAC from the left chest wall mass was performed. The smears were stained with Haematoxylin and Eosin and Leishman stains. The smears were cellular and showed discohesive sheets of predominantly monomorphic large round neoplastic cells with centrally or eccentrically placed nuclei, coarse unevenly dispersed chromatin, prominent nucleoli and moderate amount of cytoplasm, admixed with small atypical lymphoid cells and mature plasma cells. Few binucleate and multinucleated forms, atypical mitotic figures and apoptotic bodies were seen [Table/Fig-2]. A possibility of NHL plasmablastic type was the preferred diagnosis. FNAC was performed from periorbital and perianal swellings which revealed similar cytomorphology.
Photomicrograph of FNAC smear showing: a) Sheets of plasmablasts admixed with apoptotic body (arrow) (H&E stain, 40X); b) Scattered atypical lymphoid cells and tingible body macrophages (arrow) (H&E stain, 10X); c) Sheets of plasmablasts (Leishman stain, 40X); d) Scattered plasmacytoid cells and mitoses (arrow) (Leishman stain, 10X). Inset showing binucleate (short arrow) and trinucleate cells (long arrow) (Leishman stain, 40X).
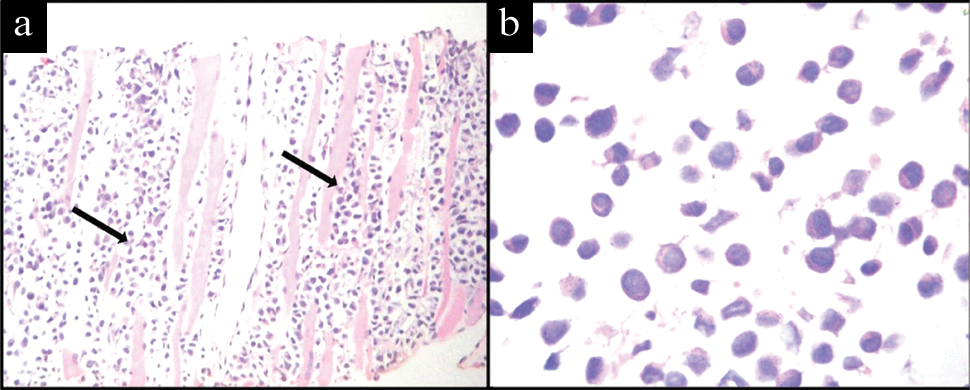
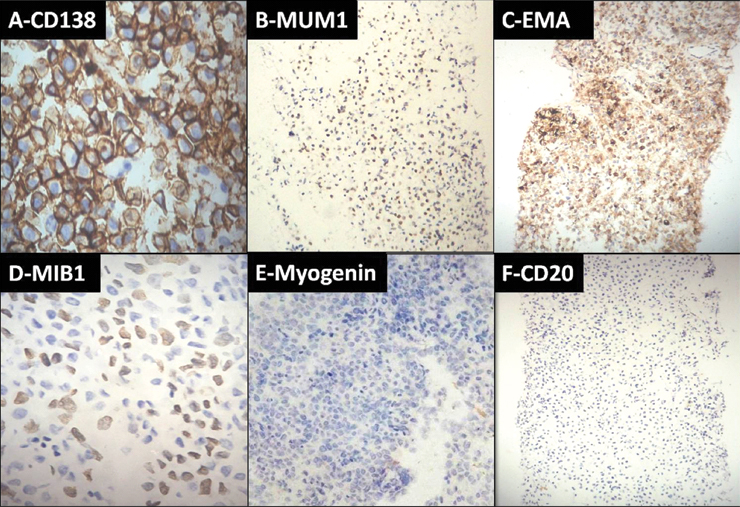
Immunocytochemistry was performed to confirm plasmablastic NHL and exclude other possible differential diagnoses such as rhabdomyosarcoma and showed weak positivity for Leukocyte Common Antigen (LCA) with strong positive staining for CD138, MUM1, EMA and a high MIB 1 proliferation index. There was negative staining for myogenin and CD20 [Table/Fig-3]. Subsequent biopsy of the chest wall swelling showed skeletal muscles infiltrated by sheets of medium sized round to oval cells with a high nucleocytoplasmic ratio, coarse chromatin admixed with plasmacytoid cells [Table/Fig-4]. Immunohistochemistry showed positivity for CD138, MUM1 and negativity for myogenin and CD20 [Table/Fig-5], thus confirming the diagnosis of PBL. The patient was started on high dose palliative chemotherapy but unfortunately did not survive.
Immunocytochemistry showing positivity for: a) CD138 (40X); b) MUM1 (10X); c) EMA (10X); d) High MIB 1 proliferation index (40X); e) Negativity for myogenin (4X) and f) CD20 (40X).

Photomicrograph showing; a) Skeletal muscle bundles (arrow) infiltrated by medium sized neoplastic cells. (H&E stain, 10X); b) Sheets of plasmablasts admixed with few plasmacytoid cells. (H&E stain, 40X)
Immunohistochemistry showing positivity for; a) CD138 (40X); b) MUM1 (4X); c) EMA (4X); d) high MIB 1 proliferation index (40X) and negativity for; e) myogenin (10X); and f) CD20 (4X).
Discussion
PBL is a subtype of B-cell NHL, initially described in HIV positive patients with a predilection for the oral cavity and jaw [1]. Other sites affected are the orbit, paranasal sinuses, anus, liver, spleen and lymph nodes. It is an aggressive lymphoma commonly arising in immunocompromised patients, but can be seen in immunocompetent persons as well [2]. PBL was first described as a specific entity associated with HIV infection by Delecluse HJ et al., [3]. Currently, it accounts for approximately 2-4% of HIV related lymphomas [4].
PBL has heterogeneous morphologic features but a distinct phenotype. The current World Health Organization classification recognizes PBL as a diffuse proliferation of neoplastic cells resembling B-immunoblasts but having the immunophenotype of plasma cells [1]. The tumour cells show weak/absent expression of Leukocyte Common Antigen LCA/CD45 and the B-cell markers CD20, CD79a, and PAX5. Instead, they express plasma cell markers CD38, CD138, and MUM1 [5].
Initial reports have described PBL in the oral cavity of HIV positive adult males [6]. It is rare in children. Literature review identified only ten cases of PBL in paediatric patients (age range: 2-15 years), with eight being HIV positive. Of these, seven were females and three were males. The sites affected were skin, oral cavity, nose, maxillary sinus, orbit, vulva, spine and scalp. The outcome was poor with few being alive postdiagnosis [6]. In our case also, the outcome was poor and our patient died within a month after starting of the chemotherapy.
FNAC is an initial investigation for the evaluation of lymphadenopathy. There are few reports in the literature that describe the cytologic findings of PBL [2,7,8]. Lin F et al., described FNAC findings of PBL from a cervical lymph node mass in an HIV negative man [8]. The aspirates showed highly atypical large tumor cells with plasmacytic differentiation admixed with lymphoid cells. In an article published by Lin O et al., three out of four patients were HIV positive with the primary sites affected being mandible, anus, stomach and cervical lymph node [7]. Cytology in all the cases showed large atypical cells with immunoblastic to plasmablastic morphology. The authors also observed multinucleated cells and mitotic figures in three cases and Tingible Body Macrophages (TBM) in one case. Immunocytochemistry was performed in only one case which showed negativity for CD45RO and CD20 whereas flow cytometry showed expression of CD10, CD38 and CD45. Reid-Nicholson M et al., described cytologic findings in five cases, two being HIV positive [2]. The most common findings were high cellularity, plasmablasts, single cell necrosis, background necrosis, and lymphoglandular bodies. The least common findings were TBM, and clusters/sheets. Bhagat VM et al., reported the cytology of a supraclavicular swelling in an HIV positive man which showed two cell populations- plasmacytoid cells and round cells with high nucleocytoplasmic ratio, coarsely granular chromatin, prominent nucleoli and scant cytoplasm along with TBM and mitoses [9]. In our case the cytology smears were cellular with scattered and sheets of plasmablasts admixed with plasmacytoid cells and lymphocytes. This characteristic morphology on FNAC in support with immunocytochemistry led us to the early diagnosis of PBL.
In view of the age and location of the tumour and cytology showing immature blastoid cells, some with eccentric nuclei, the differential diagnose of rhabdomyosarcoma and NHL with plasmacytoid differentiation were considered. However, immunocytochemistry showed negativity for myogenin and CD20 and positivity for plasma cell markers, thus favoring the diagnosis of PBL.
Plasma cell neoplasms {Extramedullary Plasmacytoma (EMP) and Plasma Cell Myeloma (PCM)} showing plasmablastic morphology may resemble PBL, both morphologically and immunophenotypically. Like PBL, EMP shows no bone marrow involvement, M-protein, or symptoms of myeloma and shows plasmacytoid differentiation on FNAC. However, PBL has strong association with HIV and EBV [9]. Unlike PBL, the myeloma cells demonstrate strong cytoplasmic K or l positivity and low MIB-1 proliferation index [8]. The detection of bone marrow involvement and serum monoclonal protein and/or Bence-Jones proteinurina, lytic bone lesions and hypercalcaemia or anaemia favors the diagnosis of PCM. In addition, CD56 and cyclin D1 may help in distinction as these markers are positive in both EMP and PCM whereas negative in PBL [7,9]. Thus, a combination of clinical, morphologic, immunophenotypic and ancillary laboratory data are necessary to distinguish PBL from blastic transformation of a plasma cell neoplasm [7,9].
PBLs with oral and extraoral manifestations represent two distinct clinicopathological entities [10]. Oral PBLs are strongly associated with HIV infection and show monomorphic plasmablastic morphology whereas extraoral PBLs tend to occur in HIV negative patients and demonstrate plasmacytic differentiation. These morphologic differences are reflected in cytology, resulting in heterogeneous cytologic findings. Our case was unusual and rare, as our patient was HIV positive presenting with extraoral manifestations but cytology showing predominantly plasmablastic morphology admixed in areas with cells of plasmacytic differentiation. The differentiated forms of PBL can be more easily diagnosed on cytology whereas poorly differentiated forms are difficult to diagnose cytologically.
Conclusion
PBL is an aggressive NHL with heterogeneous morphology but distinct immunophenotype. Although no single cytologic feature is diagnostic of PBL, a constellation of findings which include abundant immuboblasts/plasmablasts, plasma cells and atypical lymphocytes should raise suspicion and in conjunction with ancillary techniques can lead a cytopathologist to early diagnosis of PBL.
[1]. Stein H, Harris NL, Campo E, Plasmablastic lymphoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H et al, editorsWHO Classification of Tumours of Haematopoietic and Lymphoid Tissues 2008 4th EdLyon, FranceIARC Press:256-257. [Google Scholar]
[2]. Reid-Nicholson M, Kavuri S, Ustun C, Crawford J, Nayak-Kapoor A, Ramlingam P, Plasmablastic lymphoma: cytologic findings in 5 cases with unusual presentationCancer Cytopathol 2008 114(5):333-41.10.1002/cncr.2379418683216 [Google Scholar] [CrossRef] [PubMed]
[3]. Delecluse HJ, Anagnostopoulos I, Dallenbach F, Hummel M, Marafioti T, Schneider U, Plasmablastic lymphoma of the oral cavity: A new entity associated with human immunodeficiency virus infectionBlood 1997 89(4):1413-20.10.1182/blood.V89.4.14139028965 [Google Scholar] [CrossRef] [PubMed]
[4]. Khan MA, Jakate S, Komanduri S, Rare AIDS related plasmablastic lymphoma as the initial presentation of AIDSClin Adv Haematol Oncol 2010 8(1):55-57. [Google Scholar]
[5]. Castillo JJ, Reagan JL, Plasmablastic lymphoma: A systematic reviewScientific World Journal 2011 11:687-96.10.1100/tsw.2011.5921442146 [Google Scholar] [CrossRef] [PubMed]
[6]. Goedhals Stones DK, Botha MC, Plasmablastic lymphoma in childhood: a report of two casesSAJCH 2014 8(1):39-40.10.7196/sajch.613 [Google Scholar] [CrossRef]
[7]. Lin O, Gerhard R, Zerbini MCN, Teruya-Feldstein J, Cytologic features of plasmablastic lymphoma report of four casesCancer Cytopathol 2005 105(3):139-44.10.1002/cncr.2103615803491 [Google Scholar] [CrossRef] [PubMed]
[8]. Lin F, Zhang K, Quiery AT, Prichard J, Schuerch C, Plasmablastic lymphoma of the cervical lymph nodes in a human immunodeficiency virus negative patientArch Pathol Lab Med 2004 128:581-84. [Google Scholar]
[9]. Bhagat VM, Tailor HJ, Jana SH, Extra-oral plasmablastic lymphoma with pleural effusion-rare caseThe Southeast Asian Journal of Case Report and Review 2013 2(6):436-44. [Google Scholar]
[10]. Pai K, Rao L, The cytological diagnosis of extraoral plasmablastic lymphoma: A rare entityJ Clin Diagn Res 2013 7:721-22.10.7860/JCDR/2013/5759.289223730657 [Google Scholar] [CrossRef] [PubMed]