Measles, mumps and rubella are highly infectious viral diseases affecting the children early in their life and have a very high secondary attack rate [1-3]. Effective vaccination strategies coupled with sustained high vaccination coverage can reduce the risk of such highly infectious diseases and the resultant morbidity and complications [4]. The Indian Academy of Paediatrics recommends that for effective prevention of these diseases, the first dose of MMR vaccine should be given to children at the age of 9-12 months while the second dose of MMR should be given at the age of 15-18 months of age [5]. Until now the most commonly used MMR vaccine in India is Tresivac [6], manufactured by M/s Serum Institute of India Private Ltd., which contains the Edmonston Zagreb measles strain (≥ 1000 CCID50), the L-Zagreb mumps strain (≥ 5000 CCID50) and the RA 27/3 rubella strain (≥1000 CCID50). Although, the vaccine is considered to be efficacious, there is a report suggesting that there is re-emergence of mumps infection in the vaccinated population with the proposed cause of the re-emergence being the poor efficacy of mumps component in the MMR vaccine being used [7]. Moreover, MMR vaccine having the L-Zagreb mumps strain is also associated with an increased risk of aseptic meningitis [8,9].
M/s Cadila Healthcare Limited, India has developed a novel MMR vaccine (Live) (freeze-dried), containing the Edmonston Zagreb measles strain (≥1000 CCID50), the Hoshino mumps strain (≥5000 CCID50) and the RA 27/3 rubella strain (≥1000 CCID50). The Hoshino strain of mumps has been qualified by the WHO and has been used in monovalent mumps vaccine in Japan and in MMR vaccine formulations along with AIK-C measles and Takayashi rubella strains in Japan, Iran and other countries since more than 25 years [10-12]. In one of the previous reports using Hoshino mumps strain, no case of meningitis had been reported in Japan even after use of the Hoshino strain in over 7,00,000 doses in paediatric immunisation programme [11].
This MMR vaccine has been developed in two formulations i.e., a single-dose formulation (pack size of 0.5 mL to be used only for one subject) and a multi-dose formulation (pack size of 5 mL to be used for 10 subjects). Preclinical toxicology studies have been performed with this vaccine and these have not shown any specific safety concerns (data on file). Two phase I clinical trials have also been completed with the single-dose and multi-dose formulations of this novel MMR vaccine in adult healthy volunteers. Both the studies showed that the vaccine was safe and well tolerated by adult healthy volunteers (data on file).
This phase II clinical trial was conducted to evaluate the immuno-genicity and safety of the single-dose and multi-dose formulations of the novel MMR vaccine developed by M/s Cadila Healthcare Limited when administered to healthy paediatric subjects aged 15-18 months in a population routinely receiving the monovalent measles vaccine at nine months of age. The results of this phase II clinical trial paved the way for the phase III clinical trial which was a large, randomised, comparative study. The phase III study has already been completed and published at the time of preparation of this manuscript [13].
Materials and Methods
This prospective, randomised, open label, parallel group, multicentre, phase II, clinical trial was conducted at five tertiary care centres in India (GMERS Medical College and Government Hospital, Sola, Ahmedabad; Bharati Hospital and Research Centre, Bharati Vidyapeeth, Pune; GMERS Medical College and Government Hospital, Gotri, Vadodara; Padmashree Dr. D. Y. Patil Medical College and Hospital, Pune; and SSG Hospital, Vadodara) from March 2014 to August 2014. The study was conducted by paediatricians in compliance with the Indian Good Clinical Practice Guidelines and the Ethical Principles of Declaration of Helsinki [14,15]. The study was approved by the Office of the Drugs Controller General of India and was registered on Clinical Trials Registry of India (www.ctri.nic.in; CTRI/2014/01/004339). The study was started after review and approval by the Institutional Ethics Committees at each of the five participating study centres. A written informed consent was taken from the Legally Acceptable Representative (LAR) of each subject and the entire process of Informed Consent was video recorded as per the regulatory requirements of the country.
Subjects of either sex, 15 to 18 months of age, brought to the out patient department for routine MMR vaccination were enrolled in the study. Parents/guardians of the subject had to have adequate literacy to fill the diary cards for being a part of the study. Subjects were excluded from the trial if they had a history of previous measles, mumps or rubella infection or MMR vaccination; if they had been exposed to any of these three diseases within 30 days of trial commencement; or if they had received measles vaccine less than three months back. Other exclusion criteria included history of anaphylaxis or serious reactions to vaccines, hypersensitivity to neomycin, history of convulsions, epilepsy or other central nervous system diseases; an acute febrile illness at the time of enrollment in the study, history of serious chronic illness, major congenital defects, immunosuppression (immunosuppressive illness or therapy), any other clinically significant concurrent illness, administration of any other parenteral vaccine within 30 days of initiation of the study and administration of blood products or parenteral immunoglobulin during the preceding three months. Subjects were permitted to use any medication for the treatment of concomitant diseases/adverse events during the study period, not known to interact with the immunogenicity of the vaccine and a record of the same was maintained in the case record forms.
One hundred and twenty three subjects satisfying the eligibility criteria were randomised as per a centralised computer generated randomisation schedule to receive 0.5 mL single dose of the MMR vaccine either from single-dose or multi-dose formulation. The vaccine was reconstituted with water for injection (0.5 mL for single-dose formulation and 5 mL for multi-dose formulation) and was given subcutaneously in the upper arm taking aseptic precautions. Only one dose of the vaccine (0.5 mL) was used from the multi-dose formulation for the purpose of this clinical trial. The subjects were observed for at least 30 minutes post vaccination for any adverse events. Thereafter, the subjects were followed on an outpatient basis for the total duration of 42 days with scheduled visits on Day 3, Day 7, Day 14 and Day 42 post-vaccination.
One mL of blood was collected from the subjects before vaccination and 42 days after vaccination for immunogenicity assessments. The serum IgG antibody titres against the measles, mumps or rubella viruses were assessed by commercial ELISA kits manufactured by IBL International, Germany. The assay cut-offs were: 200 mIU/mL for anti-measles antibodies, 8 EU/mL for anti-mumps antibodies and 8 IU/mL for anti-rubella antibodies [16-18]. An antibody titre equal to or greater than the cut-off was defined as seropositive. In initially seronegative subjects, seroconversion was defined as appearance of antibody levels above the cut-off levels. Geometric mean of the post vaccination anti-measles, anti-mumps and anti-rubella antibody titres was calculated for all the subjects considered for immunogenicity analysis at the end of the study.
Diary cards were provided to the parents/guardians of the enrolled subjects to record solicited local (pain, tenderness, swelling, induration, ecchymosis) and systemic adverse reactions (fever, rash, rhinorrhoea and myalgia) during the first two weeks after vaccination. All the other adverse events recorded during the first two weeks after vaccination or any adverse event noted after the first two weeks were recorded as unsolicited adverse events. The adverse events were graded from Grade 1 to Grade 4 based on the severity as shown in [Table/Fig-1] (adopted from UD FDA Guidance on Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials [19]). The causality of the adverse events was assessed by investigators using the World Health Organisation Uppsala Monitoring Centre (WHO-UMC) criteria [20].
Adverse Event Grading [19].
| Reaction | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|
| Pain | Does not interfere with activity | Interferes with activity or repeated use of non-narcotic pain reliever | Prevents with activity or repeated use of narcotic pain reliever | Emergency Room (ER) visit or hospitalisation |
| Tenderness | Mild pain to touch | Pain with movement | Significant pain at rest | ER visit or hospitalisation |
| Erythema/Redness | <5 cm | 5.1-10 cm | >10 cm | Necrosis or exfoliative dermatitis |
| Swelling | <5 cm and does not interfere with activity | 5.1-10 cm or interferes with activity | >10 cm or prevents daily activity | Necrosis |
| Induration | Induration <1.0 cm in diameter | >1.0 to <2.0 cm in diameter | >2.0 to <5.0 cm in diameter | >5.0 cm in diameter |
| Ecchymosis | <2.5 cm | 2.5-5 cm | 5.1-10 cm | >10 cm |
| Fever (°C/°F)* | 38.0-38.4/100.4 –101.1 | 38.5-38.9/101.2-102.0 | 39.0-40.0/102.1-104.0 | >40/>104 |
| Rash, Rhinorrhea, Myalgia, Any other | No interference with activity | Some interference with activity | Significant, prevents daily activity | ER visit or hospitalisation |
*Axillary temperature
Immunogenicity assessments were done for both Per Protocol (PP) Population (defined as all the randomised subjects who had completed the trial as per the defined protocol without any violations with both pre and post-vaccination immunological assessments) and modified Intention to Treat (mITT) Population (defined as all the randomised subjects with both pre and post-vaccination immunological assessments including subjects with protocol violations). Immunogenicity results of PP population (114 subjects) are described here.
Statistical Analysis
Chi-Square test or Fisher’s Exact test was used to compare the immunogenicity and safety data of single-dose group with that of multi-dose group. GMTs were calculated by taking the antilog of the mean of the log transformed antibody titres. Unpaired t-test was used to compare the log transformed data of the antibody titres of the two groups. The p-values<0.05 were considered as statistically significant. As this was the first exposure of Indian paediatric subjects to the novel MMR vaccine developed using Hoshino strain of mumps virus, a sample size of only 50 evaluable subjects in each group was approved by the Regulatory Agency for this exploratory phase II clinical trial. Considering a drop out of approximately 20%, a correspondingly higher number of subjects were enrolled in the study.
Results
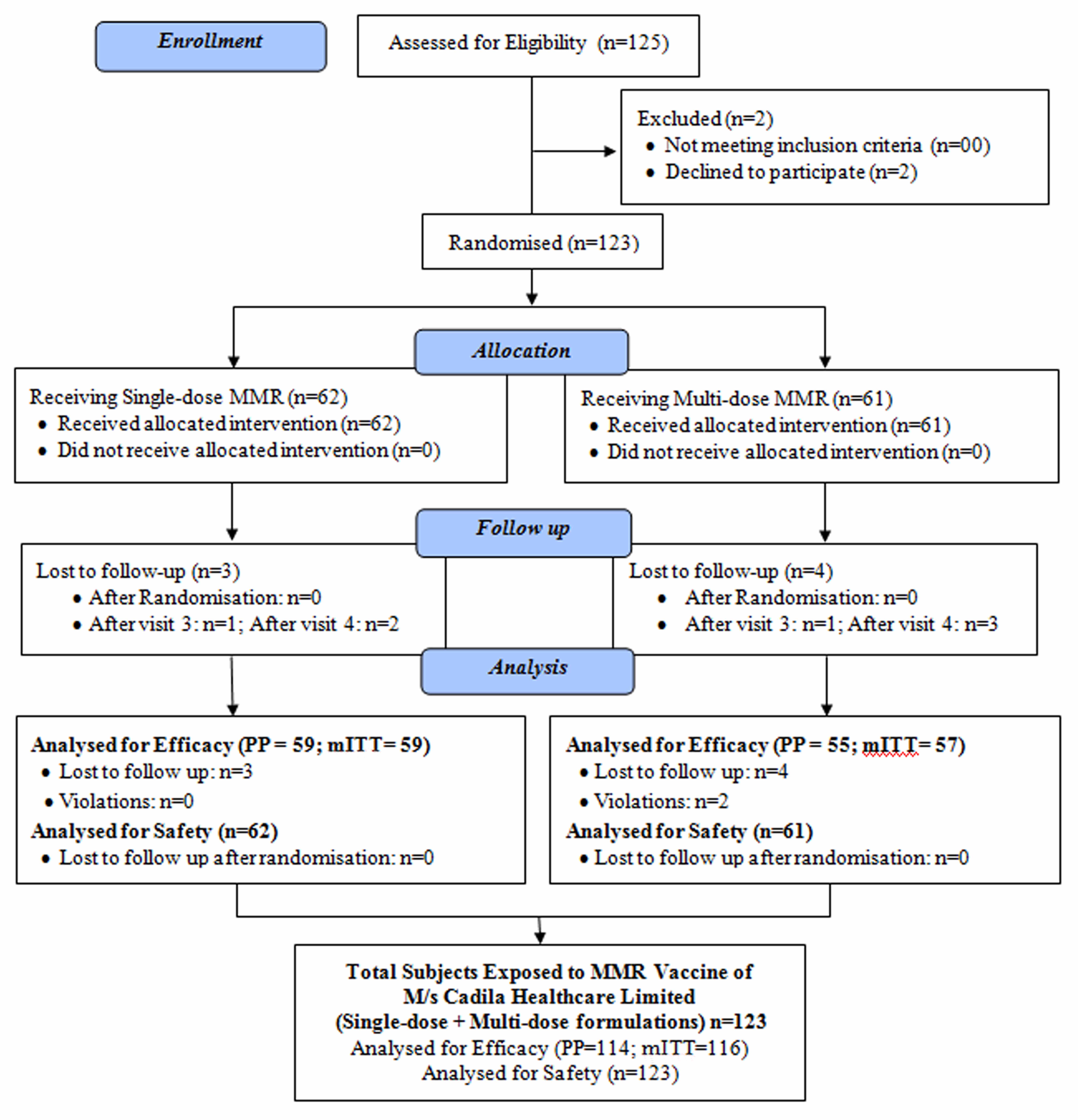
A total of 123 subjects were enrolled in this randomised, open label, multicentre phase II clinical trial. Sixty two subjects received the vaccine from the single-dose formulation while 61 subjects received it from the multi-dose formulation. A total of 114 subjects were considered for PP immunogenicity analysis and all the 123 subjects were considered for safety analysis. The flow of subjects in the study is shown in [Table/Fig-2]. The mean age of the subjects enrolled in the study was 16.6±1.2 months. The demographic and baseline characteristics of the subjects are shown in [Table/Fig-3].
Flow of subjects in the study.
Demographic and baseline characteristics of the enrolled subjects.
| Single-dose (n=62) | Multi-dose (n=61) | Total (n=123) |
|---|
| Age (Months) | 16.7±1.2(16.4–17.0) | 16.5±1.1(16.2–16.8) | 16.6±1.1(16.4–16.8) |
| Sex* | Male | 36 (58.1) | 35 (57.4) | 71 (57.7) |
| Female | 26 (41.9) | 26 (42.6) | 52 (42.3) |
| Height (cm) | 76.6±5.1(75.3–77.9) | 75.4±4.4(74.2–76.5) | 76.0±4.8(75.1–76.8) |
| Weight (kg) | 8.9±1.4(8.6–9.3) | 8.8±1.3(8.5–9.1) | 8.9±1.4(8.6–9.1) |
Data expressed as mean±SD (95% CI)
*Data expressed as n (%)
Immunogenicity
Among the 114 subjects considered for immunogenicity analysis; 22, 101 and 87 subjects were seronegative for anti-measles, anti-mumps and anti-rubella antibodies respectively at baseline. All the 22 and 101 seronegative subjects for anti-measles and anti-mumps antibodies seroconverted six weeks after the vaccination while 86 of the 87 subjects seronegative for anti-rubella antibodies seroconverted six weeks after the MMR vaccination. There was no difference in the seroconversion rates in the subjects receiving the MMR vaccine either from the single-dose formulation or the multi-dose formulation [Table/Fig-4]. The proportion of subjects seropositive and the GMT at the end of the study are shown in [Table/Fig-5,6], respectively. All the subjects considered for immunogenicity assessment had a rise in their antibody titres as compared to their baseline titres. Even in the subjects already seropositive at baseline (92, 13 and 27 for anti-measles, anti-mumps and anti-rubella antibodies respectively), approximately 85% of subjects had more than four fold rise in their anti-measles, anti-mumps and anti-rubella antibody titres.
Seroconversion rate in subjects seronegative at baseline (PP Population).
| Single-dose (n=59) | Multi-dose (n=55) | Total (n=114) |
|---|
| Measles |
| Subjects seronegative at baseline* | 13 (22.1%) | 9 (16.4%) | 22 (19.3%) |
| Seroconversion rate** | 13 (100%) | 9 (100%)# | 22 (100%) |
| Mumps |
| Subjects seronegative at baseline* | 52 (88.1%) | 49 (89.1%) | 101 (88.6%) |
| Seroconversion rate** | 52 (100%) | 49 (100%)# | 101 (100%) |
| Rubella |
| Subjects seronegative at baseline* | 42 (71.2%) | 45 (81.8%) | 87 (76.3%) |
| Seroconversion rate** | 41 (97.6%) | 45 (100%)## | 86 (98.9%) |
Data expressed as n(%)
*Proportion calculated from PP population of the group
**Proportion calculated from number of subjects seronegative at baseline
#p=1.0 (Fischer’s exact test; single-dose vs multi-dose)
##p=0.48 (Fischer’s exact test; single-dose vs multi-dose), PP-Per protocol
Proportion of subjects sero-positive at baseline and end of the study (PP Population).
| Sero-positive n (%) |
|---|
| Single-dose (n=59) | Multi-dose (n=55) | Total (n=114) |
|---|
| Measles |
| Pre-immunisation | 46 (77.9%) | 46 (83.6%) | 92 (80.7%) |
| Post-immunisation | 59 (100%) | 55 (100%) | 114 (100%) |
| Mumps |
| Pre-immunisation | 7 (11.9%) | 6 10.5%) | 13 (11.4%) |
| Post-immunisation | 59 (100%) | 55 (100%) | 114 (100%) |
| Rubella |
| Pre-immunisation | 17 (28.8%) | 10 (18.2%) | 27 (23.7%) |
| Post-immunisation | 58 (98.3%) | 55 (100%) | 113 (99.1%) |
PP-Per protocol
Post Immunization Geometric Mean Titres of Anti-measles, Anti-mumps and Anti-rubella IgG antibodies (PP Population).
| Post Immunisation Geometric Mean Titres |
|---|
| Single-dose (n=59) | Multi-dose (n=55) | Total (n=114) |
|---|
| Anti-measles antibodies (mIU/mL) | 2938.1 (2377.8–3630.3) | 3403.2* (2695.0–4297.7) | 3154.0 (2700.5–3683.6) |
| Anti-mumps antibodies (EU/mL) | 109.0 (80.6–147.4) | 74.3* (53.7–102.9) | 90.6 (72.6–113.0) |
| Anti-rubella antibodies (IU/mL) | 165.6 (115.0–238.5) | 119.8* (96.9–148.0) | 141.7 (114.4–175.4) |
Data expressed as geometric mean (95% CI)
*p>0.05 (Unpaired t-test; Single-dose Vs Multi-dose)
Immunogenicity was analysed in the mITT population also (116 subjects) and it was found that the seroconversion rate, the proportion of subjects seropositive after vaccination and the GMTs were similar to that in the PP population.
Safety
In the 123 subjects analysed for safety, a total of 43 adverse events were recorded in 28 subjects, giving an adverse event rate of 21.8% in the study. Twenty seven adverse events were recorded in 17 subjects who had received the MMR vaccine from the Single-dose formulation while 16 adverse events were recorded in 11 subjects who had received the vaccine from the multi-dose formulation (the difference was not statistically significant; p=0.31). Most of the adverse events reported in the study, 41 out of 43 events (95.3%) were of Grade I severity. No serious adverse event was reported in any of the subjects enrolled in the study. All the reported adverse events resolved completely with/without symptomatic treatment. The mean duration of adverse events was 2.7 days. While most of the adverse events resolved within one to three days (31 out of 43 events; 72.1%), 12 events resolved within four to seven days of occurrence of events.
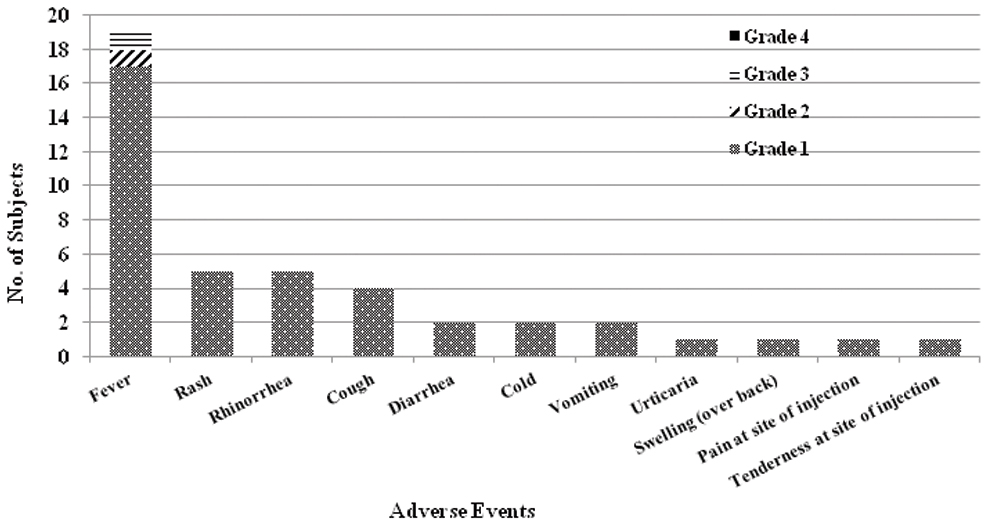
The most common adverse event reported during the study was fever in 19 subjects (15.4%) followed by rash and rhinorrhoea in five subjects (4.1%) each. The incidence of fever peaked in the first two weeks after vaccination (17 out of 19 cases; 89.5%). Most of the subjects (17 out of 19; 89.5%) had Grade 1 (38.0-38.4°C) fever only, while one subject each developed Grade 2 (38.5-38.9°C) and Grade 3 fever (39.0-40.0°C) and none of the subjects developed Grade 4 fever (>40°C). The other adverse events recorded during the study, irrespective of their causal relation to the vaccine are shown in [Table/Fig-7]. Among the 43 adverse events reported during the study, 35 events (81.4%) were considered to have a ‘probable’ association with the study vaccine while eight events (18.6%) were ‘unlikely’ to be related to the study vaccine. Among these eight events, there were three cases of fever (2.4%) and one case each of cough (0.8%), cold (0.8%), diarrhoea (0.8%), urticaria (0.8%) and swelling (0.8%).
Adverse events reported post MMR vaccination.
Discussion
This study presents the results of the first study in Indian paediatric subjects exposed to the novel MMR vaccine having the Hoshino mumps strain. The immunogenicity results of the study show that both the single-dose and multi-dose formulations of the novel MMR vaccine are immunogenic having seroconversion rates of 100% for anti-measles and anti-mumps antibodies and; 98.9% for anti-rubella antibodies. The seroconversion rates found in our study are comparable to those found in the published literature [21,22]. In one of the studies assessing the MMR vaccine of SmithKline Beecham Biologicals (Priorix; containing the Schwarz measles virus strain, the RIT 4385 strain of mumps virus and the Wistar RA 27/3 rubella virus strain) in over 1000 children aged nine to 24 months, the seroconversion rate was found to be 98.7% for anti-measles antibodies, 95.5% for anti-mumps antibodies and 99.5% for anti-rubella antibodies [21]. In another study comparing the GlaxoSmithKline MMR vaccine (containing the Schwarz measles virus strain, the RIT 4385 strain of mumps virus and the Wistar RA 27/3 rubella virus strain) with that of Merck MMR (containing the Enders Edmonston measles virus strain, the Jeryl Lynn strain of mumps virus and the RA 27/3 rubella virus strain) in about 225 children aged between 12 and 18 months who had received the monovalent Measles vaccine at nine months of age, the seroconversion rates in subjects seronegative at baseline were found to be 100% for anti-measles and anti-rubella antibodies for both the vaccines and; 93.5% and 97.9% for anti-mumps antibodies for the GlaxoSmithKline MMR and the Merck MMR vaccine, respectively [22]. The seroconversion rates with MMR vaccine of Serum Institute of India (containing Edmonston-Zagreb measles virus, Leningrad-Zagreb mumps virus and Wistar RA 27/3 rubella virus) have been found to be 100% for anti-measles antibodies, 99% for anti-rubella antibodies and 92% for anti-mumps antibodies in Indian paediatric subjects aged 18 to 24 months who have had a previous monovalent measles vaccine at 9 months of age [23]. All these studies have shown consistent seroconversion rates for anti-measles and anti-rubella antibodies but have shown to have a variable response for anti-mumps antibodies. It has been shown that the development of antibodies against a virus depends on the viral strain used in the vaccine. All the MMR vaccines quoted above have different strains of mumps virus in their formulations and this could be the reason for the variable seroconversion for anti-mumps antibodies. The MMR vaccine of M/s Cadila Healthcare Limited contains the Hoshino strain of the mumps virus and has shown to have a higher seroconversion rate as compared to the published data of seroconversion of anti-mumps antibodies with other strains [21,22]. The results of this study are in line with the results of a clinical study in Japan, wherein the seroconversion for anti-mumps antibodies was found to be 96.8% with the MMR vaccine having the Hoshino mumps strain and 100% with monovalent Hoshino mumps vaccine [11]. Besides the effect on humoral immunity, the Hoshino mumps strain has also shown to induce cellular immunity in over 90% of the recipients, as judged by the virus specific interferon-γ production [24].
The safety profile of this MMR vaccine is also found to be good. The two different formulations of the MMR vaccine i.e., single-dose and multi-dose (10 dose vial) formulation used in this study had the same concentration of the virus strains per dose and there was no difference in the safety profile of the two formulations. The vaccine was well tolerated by all the subjects and none of them had any serious adverse event during the study period. The most common adverse event reported during the study was fever in 19 subjects (15.4%) followed by rash and rhinorrhoea in five subjects (4.1%) each. The safety profile of this vaccine is comparable to that of the other MMR vaccines available internationally and in India [21-23]. In an Indian study assessing the immunogenicity and reactogenicity of an indigenously produced MMR vaccine, fever (5.9%) was the most common adverse event followed by cough (4.6%) and cold (3.2%) [23]. In international studies also, the most common adverse events reported with MMR vaccine are fever followed by rash [21,22]. The safety of the Hoshino mumps strain containing vaccines is also well established as seen in the various clinical trials and post marketing surveillances conducted during use in national immunisation programmes, internationally [11,12].
Limitation
One of the limitation of this phase II study was the small sample size. Moreover, this was an uncontrolled study and all the comparisons made in this manuscript are based on the published literature wherein the historical studies have varied designs and are performed under various settings using different ELISA kits for immunological assessments.
Conclusion
The results of this phase II clinical trial are encouraging and have shown that the novel MMR vaccine (live) (freeze-dried) developed by M/s Cadila Healthcare Limited is immunogenic and well tolerated by healthy paediatric subjects aged 15-18 months. The results of this study suggest that the vaccine should be taken forward for a large, randomised, comparative, phase III study.
Financial support: This study was sponsored and funded by Cadila Healthcare Limited, India. Authors 1-5 received honoraria for conduct of the study. Authors 6 and 7 are employees of Cadila Healthcare Limited.
*Axillary temperature
Data expressed as mean±SD (95% CI)
*Data expressed as n (%)