Cushing Syndrome: Secondary to Squamous Cell Carcinoma of the Lung
Vishnu Vardhan Garla1, Christine Oakley2
1 Assistant Professor, Department of Internal Medicine, University of Mississippi Medical Centre, Marshall University School of Medicine, Huntington, USA.
2 Consultant Endocrinologist, Department of Internal Medicine, St. Vincnet’s Medical Group, Marshall University School of Medicine, Huntington, USA.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Vishnu Vardhan Garla, 2500 North State Street, Jackson, Mississippi-39217, USA.
E-mail: vgarla@umc.edu
Cushing syndrome secondary to ectopic Adrenocorticotrophic (ACTH) hormone secretion is an uncommon entity mostly caused by small cell lung cancer or bronchial carcinoids. It can be characterised by absence of classical cushingoid features. Ectopic ACTH syndrome is characterised by severe and progressive hypercortisolemia. Thus, prompt identification and treatment can significantly decrease morbidity in affected patients. A 57-year-old male patient with a history of Stage 4 squamous cell cancer of the lung presented with worsening hyperglycemia and hypokalemia. Physical exam was notable for facial plethora and hypertension. Hormonal testing showed a very high serum cortisol, ACTH and 24 hours urinary free cortisol levels. MRI of the brain was normal. A diagnosis of ectopic ACTH syndrome was confirmed. The patient was started on Ketoconazole and his cortisol levels improved. The patient was discharged and eventually was lost to follow up.
Ectopic adrenocorticotrophic syndrome, Hypercortisolemia, Hypokalemia, Non-small cell lung cancer
Case Report
A 57-year-old male with history of hypertension, coronary artery disease, prior tobacco abuse, and Stage 4 squamous cell cancer of the lung was admitted to the hospital for evaluation of worsening hypertension and new-onset hypokalemia. He was diagnosed with Stage 4 squamous cell carcinoma of the lung two years prior to presentation. He was initially treated with Carboplatin and Paclitaxel followed by cyberknife radiotherapy. After completion of radiotherapy patient was put on maintenance with Erlotinib therapy. After 20 months the patient’s Positron Emission Tomography (PET) scan showed new lymph node enlargements raising the suspicion of metastasis. The patient was then started on Gemcitabine and Carboplatin. On presentation physical examination showed a blood pressure of 171/95 mmHg and facial plethora. Laboratory assessment showed a serum potassium of 2.8 mmol/L (3.1-5.5 mmol/L), magnesium of 1.4 mg/dL (1.8-2.4 mg/dL), serum creatinine of 1.0 mg/dL (0.5-1.3mg/dL), glucose of 147mg/dL (70-100 mg/dL) and plasma cortisol of 43.6 mcg/dL (5-25 mcg/dL). Intravenous potassium repletion was initiated, but the patient remained markedly hypokalemia. He was also noted to have elevated blood glucose level over 200 mg/dL, particularly by later in the day. Endocrinologist was consulted for evaluation of suspected Cushing syndrome. Further hormonal testing [Table/Fig-1], was notable for hypercortisolemia and elevated serum ACTH. In order to localise the source of ACTH, MRI of the brain and pituitary was done which showed no abnormalities of the pituitary gland. A high dose dexamethasone (8 mg dexamethasone) suppression test did not result in suppression of the cortisol levels. The results of the MRI brain and the high dose dexamethasone suppression test confirmed the diagnosis of ectopic ACTH syndrome. The source of ACTH was suspected to be the patient’s squamous cell carcinoma of lung [Table/Fig-2]. The patient was treated with Ketoconazole (200 mg three times a day). Daily morning cortisol levels decreased from 69.2 mcg/dL to 41.8 mcg/dL after initiation of treatment with Ketoconazole. The patient was discharged but was lost to follow up.
Laboratory tests done on admission to the hospital.
| S.No | Test | Result |
|---|
| 1. | Plasma renin (0.15-2.33 ng/mL/hour) | <0.15 |
| 2. | Plasma aldosterone (0-30 ng/mL) | 1 |
| 3. | Urinary metanephrines (0-62 pg/mL) | 30 |
| 4. | Urinary normetanephrines (0-145 pg/mL) | 51 |
| 5. | Plasma ACTH (7.2-63.3 pg/mL) | 202.6 |
| 6. | Plasma cortisol (5-25 mcg/dL) | 39 |
| 7. | Salivary cortisol (0.025-0.6 mcg/dL) | 3.9 |
| 8. | 24 hr urinary free cortisol (0-50 mcg/24 hours) | 15.496 |
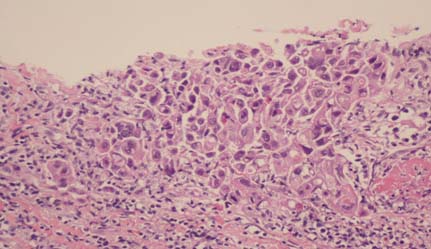
Biopsy of the lung (200X magnification) showing characteristic intercellular bridges of squamous cell carcinoma of the lung.
Discussion
Ectopic ACTH syndrome comprises about 20% of ACTH-dependent Cushing syndrome cases. The most common causes of ectopic ACTH syndrome are bronchial carcinoids and small cell carcinoma of the lung. In addition, various tumours like thymic carcinoids, pancreatic carcinoids, medullary carcinoma of the thyroid, gastrinoma mesothelioma, lymphoma, leukemia and pheochromocytoma can cause ectopic ACTH syndrome [1]. A non small cell lung cancer causing ectopic ACTH syndrome is a very rare occurrence. Our literature search revealed only two previous reports of squamous cell cancer of the lung causing ectopic ACTH syndrome [2,3].
Ectopic ACTH syndrome is further classified into overt when the tumoural source is easily detected on initial tests and covert when the tumoural source was not discovered in the initial tests. Occult ectopic ACTH syndrome is when no tumoural source can be found but all the laboratory tests point towards an ectopic cause [1].
We hypothesise that in our patient the initial response to chemotherapy lead to selection chemo resistant cell clones which secreted ACTH. These cell clones proliferated and caused chemo resistance and ectopic ACTH syndrome. Cushing Syndrome secondary to Ectopic ACTH Syndrome (CS-EAS) has been known to occur during relapse of small cell cancer of the lung [4].
CS-EAS has wide variability in clinical presentation. The average age of presentation is about a decade later when compared to Cushing disease. The most common clinical features are worsening hypertension, hyperglycemia and hypokalemia as seen in our patient. Weight gain is not a universal finding; in fact, weight loss has been reported in 10 to 21% of patients in different case series [5]. Other clinical features including hyperpigmentation, weakness secondary to proximal myopathy and psychiatric symptoms may be present. The clinical manifestations depend on the degree of hypercortisolemia rather than the source of ACTH; however, small cell lung cancer patients may have a more rapid onset of symptoms when compared to neuroendocrine tumours [1].
A comparative account of various laboratory indices and imaging studies in adrenal, pituitary and ectopic Cushing’s syndrome is detailed in [Table/Fig-3] [6]. Management of ectopic ACTH syndrome is surgical excision when a single source has been located however, this may not be possible in patients with extensive disease. In our case metastatic disease ruled out any surgical intervention. Adrenolytic medications like ketoconazole and metapyrone are relatively safe in treating hypercortisolemia. Their dose should be titrated to achieve normal levels of cortisol. Replacement with glucocorticoids may be needed after adequate adrenal suppression is achieved [7].
Comparison of adrenal, pituitary and ectopic cushing syndrome.
| Test | Adrenal Cushing syndrome | Pituitary Cushing disease | Ectopic Cushing syndrome |
|---|
| ACTH (7.2-63.3 pg/mL) | Undetectable | >20 | Very high maybe >200 but can have overlapping values with Cushing disease |
| Salivary cortisol (0.025-0.6 mcg/dL) | Increased | Increased | Very high |
| 24 hours urinary free cortisol | Increased | Increased | Very high |
| Low dose dexamethasone suppression test | Not suppressed | Not suppressed | Not suppressed. |
| CRH stimulation tests | No response | Response | No increase in ACTH however 10-15 % cases have been shown to respond |
| MRI Brain | Normal | Pituitary adenomas detected in 50% cases | Normal |
| Bilateral inferiorpetrosal sinus sampling | Not applicable | Gradient observed | No gradient observed |
ACTH – Adrenocorticotrophic hormone, CRH-Cortisol releasing hormone, MRI-Magnetic resonance imaging.
Patients with Cushing’s syndrome are at risk for various complications like infection and venous thromboembolism. Previous studies have shown the risk of infections to be directly related to the degree of hypercortisolemia. Glucocorticoids may also mask the manifestations of infection leading to a delayed diagnosis which may lead to increased mortality and morbidity [8]. Patients with Cushing syndrome also have elevated levels of factors II, V, VIII, IX, XI and XII putting them at a higher risk of thrombosis [9]. Theoretically, patients with ectopic ACTH syndrome may be at a higher risk for thromboembolism, as malignancy is also a known risk factor for thromboembolism [5].
The prognosis depends on the primary tumour causing ectopic ACTH syndrome. Patients with small cell cancer lung cancer have worse prognosis as compared to patient with neuroendocrine tumours who have a better prognosis [1].
In summary, Cushing syndrome due to ectopic ACTH syndrome can occur secondary to various types of tumours. Such cases can present early on, or be noted to develop later in those with known malignancy diagnoses. Affected patients may present with hypokalemia, hypertension, and hyperglycemia related to hypercortisolemia. There is an increased incidence of infectious and thrombotic complications in these patients which may result in a worse prognosis. Early detection and appropriate management of this condition requires a multidisciplinary approach, which may result in a better prognosis.
Conclusion
To our knowledge this is only the third case of squamous cell cancer of the lung causing Cushing’s syndrome. Ectopic Cushing’s syndrome is characterised by severe and progressive hypercortisolemia; thus, prompt identification and treatment of this condition can significantly decrease morbidity in affected patients.
ACTH – Adrenocorticotrophic hormone, CRH-Cortisol releasing hormone, MRI-Magnetic resonance imaging.
[1]. Alexandraki KI, Grossman AB, The ectopic ACTH syndromeRev Endocr Metab Disord 2010 11(2):117-26.10.1007/s11154-010-9139-z20544290 [Google Scholar] [CrossRef] [PubMed]
[2]. Noorlander I, Elte JW, Manintveld OC, Tournoy KG, Praet MM, van Meerbeeck JP, A case of recurrent non-small-cell lung carcinoma and paraneoplastic Cushing’s syndromeLung Cancer 2006 51(2):251-55.10.1016/j.lungcan.2005.08.01516352372 [Google Scholar] [CrossRef] [PubMed]
[3]. Imura H, Matsukura S, Yamamoto H, Hirata Y, Nakai Y, Endo J, Studies on ectopic ACTH-producing tumours. II. Clinical and biochemical features of 30 casesCancer 1975 35(5):1430-37.10.1002/1097-0142(197505)35:5<1430::AID-CNCR2820350529>3.0.CO;2-O [Google Scholar] [CrossRef]
[4]. Suyama K, Naito Y, Yoh K, Niho S, Goto K, Ohmatsu H, Development of Cushing’s syndrome during effective chemotherapy for small cell lung cancerIntern Med 2011 50(4):335-38.10.2169/internalmedicine.50.412721325767 [Google Scholar] [CrossRef] [PubMed]
[5]. Ejaz S, Vassilopoulou-Sellin R, Busaidy NL, Hu MI, Waguespack SG, Jimenez C, Cushing syndrome secondary to ectopic adrenocorticotropic hormone secretion: the University of Texas MD Anderson Cancer Center ExperienceCancer 2011 117(19):4381-89.10.1002/cncr.2602921412758 [Google Scholar] [CrossRef] [PubMed]
[6]. Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP, Diagnosis and complications of Cushing’s syndrome: a consensus statementJ Clin Endocrinol Metab 2003 88(12):5593-02.10.1210/jc.2003-03087114671138 [Google Scholar] [CrossRef] [PubMed]
[7]. Isidori AM, Lenzi A, Ectopic ACTH syndromeArq Bras Endocrinol Metabol 2007 51(8):1217-25.10.1590/S0004-2730200700080000718209859 [Google Scholar] [CrossRef] [PubMed]
[8]. Sarlis NJ, Chanock SJ, Nieman LK, Cortisolemic indices predict severe infections in Cushing syndrome due to ectopic production of adrenocorticotropinJ Clin Endocrinol Metab 2000 85(1):42-47.10.1210/jc.85.1.4210634361 [Google Scholar] [CrossRef] [PubMed]
[9]. Kastelan D, Dusek T, Kraljevic I, Polasek O, Giljevic Z, Solak M, Hypercoagulability in Cushing’s syndrome: the role of specific haemostatic and fibrinolytic markersEndocrine 2009 36(1):70-74.10.1007/s12020-009-9186-y19381886 [Google Scholar] [CrossRef] [PubMed]