Mucormycosis is an opportunistic invasive fungal infection caused by Mucorales of class Zygomycetes. It is commonly seen in immunosuppressed cases. Gastrointestinal infection (GI) is very uncommon among adults and is diagnosed only on histopathological examination of biopsy or resection specimens. Due to non-specific clinical presentation, intestinal mucormycosis is diagnosed late. We report a series of six cases of intestinal mucormycosis in adults.
Three years retrospective analysis of ileal and colonic resection specimens was performed in our tertiary care institute. All the cases had mucosal ulceration and necrosis on microscopic examination and one case also exhibited angioinvasion. Histopathology is the cornerstone of diagnosis and prompt management of invasive mucormycosis which may follow a rapid and fatal course in the absence of timely and optimum management. The clinicians need to keep the possibility of this vasculotropic fungus in mind in all cases presenting with abdominal symptoms.
Fungal disease, Gut, Infectious, Zygomycosis
Mucormycosis is an opportunistic invasive fungal infection caused by Mucorales of class Zygomycetes. Predisposing conditions include malnutrition, carcinoma, uraemia, haematological malignancies, abdominal trauma, desferoxamine therapy. In adults, mucormycosis commonly occurs as rhinocerebral (39%), pulmonary (22%), cutaneous (16%), disseminated forms (16%) or GI (4%) infection. GI is very uncommon and is commonly seen in malnourished infants. Previously, Gastrointestinal Tract (GIT) mucormycosis was seen in 4-7% of all cases. However, there has been a rise in the incidence of GIT involvement by mucor in adults.
We present here six cases of intestinal mucormycosis infection in adults which was diagnosed only after histopathological examination of the resection specimens.
Case Series
Retrospective analysis of patients who had undergone intestinal resection for non-neoplastic lesions in our tertiary care institute over a period of three years (July 2013 to June 2016) was performed. The Haematoxylin and Eosin (H&E) stained sections were studied and special stains like Periodic Acid-Schiff (PAS) and Gomori Methanamine Silver (GMS) were performed as required. The patients clinical details were obtained from the clinical records department.
Mucormycosis was seen in six out of 291 intestinal specimens resected for non-malignant causes. The mean age of the patients was 37.8 years with male preponderance (M:F ratio =2:1). Abdominal pain was present in all the cases while malaena was observed in three patients [Table/Fig-1]. Weight loss and fever were noted in two cases each. One case was a diabetic patient since 15 years who presented with vague abdominal pain and distension and was suspected to develop intestinal gangrene. Another case came with Aluminium Phosphide poisoning and was clinically suspected to have gangrene of large and small intestine.
| S No. | Age (years)/sex | Clinical features | Intraoperative findings | Segment involved | Gross findings | Microscopy | Mucor | Course and Treatment |
|---|
| 1. | 68/M | Abdominal pain, melaena, Colon Ulcer | Perforation | Colon | Serositis, perforation | Ulcerated mucosaIschaemic necrosis, congested blood vessels, serositis | + | Liposomal Amphotericin B 5 mg/kg iv, three months course of itraconazole orally. Recovered |
| 2. | 33/F | Fever, abdominal pain, melaena, weight loss | Multiple Perforations | Ileum and colon | Serositis, perforations | Ulcerated mucosaIschaemic necrosis, congested blood vessels, serositis | + | Liposomal Amphotericin B 5 mg/kg iv three months course of itraconazole orally. Recovered |
| 3. | 20/F | Abdominal pain, Obstipation, Gangrenous colon | - | Ileum | Blackened intestine, serositis | Ulcerated mucosaIschaemic necrosis, congested blood vessels angioinvasion by mucor | + | Liposomal Amphotericin B 10 mg/kg daily. Recovered |
| 4. | 23/M | Abdominal pain, melaena, weight loss | - | Ileum and colon | Blackened, thinned out intestine | Necrosed mucosaIschaemic necrosis, congested blood vessels, serositis | + | Liposomal Amphotericin B 5 mg/kg iv, three months course of itraconazole orally. Recovered |
| 5. | 25/M | Abdominal pain Celphos poisoning | - | Ileum | Serositis | Ulcerated mucosaIschaemic necrosis, congested blood vessels | + | Died within one day of intestinal resection, before diagnosis |
| 6. | 58/M | Fever, abdominal pain, diabetic, obstipation,perforation | Faecal peritonitis with gangrenous gut | Colon | Serositis | Ulcerated mucosaIschaemic necrosis, congested blood vessels, serositis | + | Died within one day of intestinal resection, before diagnosis |
Perforation was noted in two cases out of six (33%) and X-ray abdomen of one of these patients showed air fluid levels; suggestive of perforation. Two cases underwent intestinal resection as they were suspected to have colonic ulcers on colonoscopy done outside. None of the cases had radiological evidence supporting any infectious pathology.
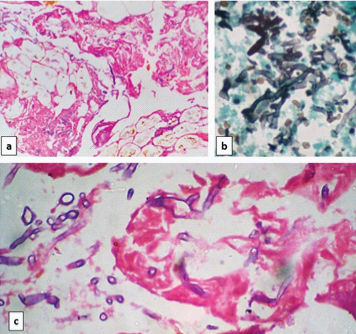
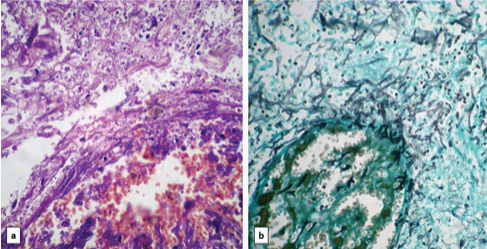
Microscopically, all cases showed mucosal ulcerations with foci of ischaemic necrosis [Table/Fig-2]. In the necrotic debris, broad aseptate fungal hyphae were identified which were morphologically consistent with mucor and highlighted on PAS and Gomori Methanamine Stains (GMS) [Table/Fig-3,4,5 and 6]. The lamina propria showed acute on chronic inflammatory infiltrate in five cases while one case showed necrosis. In two cases, foreign body giant cells were also present. None of the cases showed well-formed epithelioid granulomas. In a single case, submucosal blood vessels showed evidence of angioinvasion by the fungal hyphae [Table/Fig-7]. Serositis and haemorrhage was seen in five cases each.
Photomicrograph of case 4 shows: a) ischaemic necrosis of all layers of colon (H&E, 10X); b) Inset shows GMS stain highlighting aseptate hyphae of Mucor (40X).
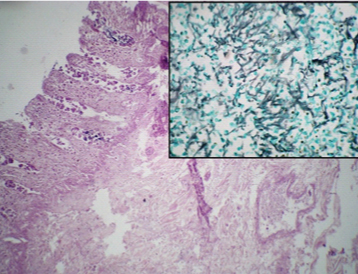
Photomicrograph of case 1 showing ribbon like broad aseptate hyphae of Mucor: black arrows on H&E stain (40X), inset-GMS stain (40X).
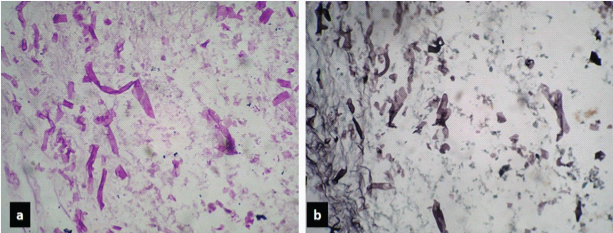
Photomicrograph of case 2 showing ribbon shaped broad aseptate hyphae of Mucor, against necrotic background on: a) PAS; and b) GMS stain (40X).
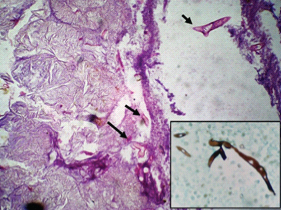
Photomicrograph of case 5 shows Mucor (black arrows) against foci of lysed fat (H&E, 40X). Inset shows hyphae of Mucor highlighted on GMS stain (40X).
Photomicrograph of case 6 showing hyphae of mucor in areas of serositis on: a) H&E stain (10X); b) GMS stain (40X); c) H&E stain (40X).
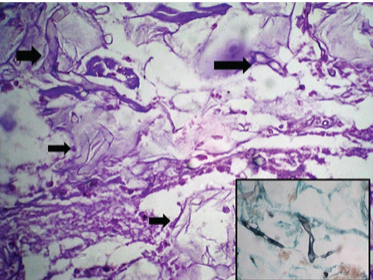
Photomicrograph of case 3 showing Mucor present in necrotic areas and destroying blood vessel wall on: a) H&E stain and b) GMS stain (40X).
Discussion
Mucormycosis is an invasive fungal infection caused by mucorales of class phycomycetes which are aerobic saprophytes present in soil and decaying organic material [1,2]. They produce spores which get airborne and enter the body through inhalation or ingestion of fomites contaminated with spores. Thus, sporangiospores are the infective forms which give rise to the angioinvasive hyphae in the human host, resulting in fulminant infections affecting various systems, including GIT, even in immunocompetent individuals [3,4]. These hyphae then invade the tissues and spread to other sites through blood vessels [4,5]. Complex interactions between iron and fungal spores determine the rate of replication and survival of fungi in vivo. These fungi scavenge free iron from the host using low molecular weight iron chelators (siderophores) or high affinity iron permeases such as ferrizoferrin [4]. Infection is commonly seen in cases having systemic immunosuppression like diabetes, malnutrition, carcinoma, uraemia, haematological malignancies, abdominal trauma, pica and patients undergoing haemodialysis who also receive desferoxamine [1,2,4,6].
Sites infected are cutaneous, pulmonary, rhinocerebral, GIT or disseminated [7]. In immunocompetent patients, other uncommon sites of mucormycotic infections like necrotising fasciitis and pericarditis have also been reported [8,9]. GI infection is uncommon but may occur due to swallowing of infected sputum or through haematological dissemination [2,4]. The infection is established due to breakdown of intestinal mucosal barrier or by local damage by surgical procedure or by disease itself [10]. Although all the parts of the GIT are prone to infection, yet stomach is affected in majority of cases followed by ileum and colon [3,5]. In this series, two cases had mucormycosis in ileum only; two cases had mucormycosis in colon only and two cases harboured mucormycosis in ileum as well as colon.
Gastrointestinal mucormycosis is diagnosed late because of non-specific clinical presentation which includes abdominal pain, nausea, vomiting, abdominal distension, melaena, frank GI bleeding, diarrhoea and fever. These features may arouse the suspicion of an intra-abdominal abscess [4,10-12]. GI mucormycosis presents as necrotizing enterocolitis in premature neonates and follows a fulminant course if not treated aggressively. Only one–fourth of the cases are diagnosed antemortem [6]. All our cases had abdominal pain while melaena was observed in three cases and two cases had fever. One of the cases also had intestinal perforation. Perforations are commonly associated with gastric infection but have also been reported in intestinal infection [4,10,12,13].
There are no specific biomarkers to detect mucormycosis. Even serological analysis for beta-D-glucan and galactomannan fails to detect cell wall antigens [14]. Endoscopy shows ulcerated plaques having raised edges and covered with black necrotic slough [15].
Grossly, the large ulcers with rolled up margins which are irregular at places are seen. Sometimes perforation occurs [16]. Microscopic features show 10-20 μm broad, ribbon like, aseptate hyphae which branch randomly at various angles [2,10,17]. These fungi are vasculotropic. Hence, angioinvasion resulting in acute vasculitis and thrombosis followed by extensive areas of tissue infarction and necrosis is the usual course of events. Angioinvasion was observed in one of our cases. Venous invasion by the fungal hyphae results in haemorrhage [11,12,17]. Mucosal ulceration with ischaemic necrosis was present in all of our cases while serositis and haemorrhage could be demonstrated in five cases. Special stains like PAS and GMS will highlight the fungal hyphae and angioinvasion can be made out easily on these special stains. Culture studies will just demonstrate the presence of the fungus but cannot tell about status of tissue infarction or vascular invasion. Histopathological examination of specimens is mandatory to identify the fungus, determine angioinvasion and tissue infarction [12,17]. No culture studies or microbiological examination was done in our cases.
Early diagnosis and treatment based on removal of the predisposing factors, administration of systemic antifungal therapy along with surgical resection of the involved gut segment can save the patients in this aggressive fungal infection [4,13,14,16,17]. Thrombosis and necrosis cause poor penetration of the drug to the tissues. Therefore, surgical debridement of the necrotic intestinal segment is essential for complete treatment [6]. Liposomal Amphotericin B (upto 10 mg/kg/day) is the drug of choice in most cases because of greater efficacy, central nervous system penetration and lesser toxic adverse effects [4]. Patients who receive immunosuppressive therapy may also be administered secondary antifungal prophylaxis [17]. Some studies have explored the efficacy of Deferiprone which inhibits uptake of iron by Rhizopus and hyperbaric oxygen therapy [4]. Posaconazole is also being evaluated as the preferred antifungal in refractory infection and patients who are intolerant to polyenes [18-20]. Other agents like recombinant cytokines including colony stimulating factors (G-CSF, GM-CSF) and interferons (IFN-gamma) are also being explored for adjunctive treatment [19]. The antifungal therapy is administered as long as the clinical signs and symptoms or radiological evidence of underlying fungal infection or the phase of immunosuppression persists [5].
Conclusion
We hereby report six cases of intestinal mucormycosis in adults. With increasing incidence of immunosuppression in patients because of diabetes, corticosteroids and immunosuppressive therapy for transplants, the incidence of fungal infections is increasing and now it is also seen in immunocompetent patients harbouring no risk factor of acquiring fungal infection. Thus, the faint possibility of fungal infection should also be kept in mind while investigating any patient presenting with abdominal symptoms, refractory to antibacterial treatment for prompt and optimum management.
[1]. Agarwal K, Sharma M, Singh S, Jain M, Antemortem diagnosis of gastrointestinal mucormycosis in neonates: Report of two cases and review of literatureIndian J Pathol Microbiol 2006 49(3):430-32.10.4103/0377-4929.4255217001912 [Google Scholar] [CrossRef] [PubMed]
[2]. Sellapan B, Bakhshi S, Safaya R, Gupta AK, Arya LS, Invasive colonic mucormycosis in early induction therapy of childhood Acute Lymphoblastic LeukemiaIndian J Pediatrics 2005 72(1):77-79.10.1007/BF0276058715684455 [Google Scholar] [CrossRef] [PubMed]
[3]. Kudva R, Kudva A, Mucormycosis of intestine in an immunocompetent individualInt J Sci Res Publ 2014 4(3):1-3. [Google Scholar]
[4]. Shiva Prasad BN, Shenoy A, Nataraj KS, Primary gastrointestinal mucormycosis in an immunocompetent personJ Postgrad Med 2008 54(3):211-13.10.4103/0022-3859.4180518626171 [Google Scholar] [CrossRef] [PubMed]
[5]. Martinello M, Nelson A, Bignold L, Shaw D, “We are what we eat!” Invasive intestinal mucormycosis: A case report and review of the literatureMedical Mycology Case Reports 2012 1:52-55.10.1016/j.mmcr.2012.07.00324371738 [Google Scholar] [CrossRef] [PubMed]
[6]. Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Epidemiology and outcome of zygomycosis: a review of 929 reported casesClinical Infectious Diseases 2005 41(5):634-53.10.1086/43257916080086 [Google Scholar] [CrossRef] [PubMed]
[7]. Lee JH, Ha HK, Yoo E, Yang SK, Min YI, Auh YH, CT and sonographically guided biopsy in a patient with intestinal mucormycosisAm J Roentgenol 2000 175(1):129-31.10.2214/ajr.175.1.175012910882261 [Google Scholar] [CrossRef] [PubMed]
[8]. Jain D, Kumar Y, Vashista RK, Rajesh L, Pattari SK, Chakrabarti A, Zygomycotic necrotizing fascitis in immunocompetent patients: A series of 18 patientsMod Pathol 2006 19(9):1221-26.10.1038/modpathol.380063916741524 [Google Scholar] [CrossRef] [PubMed]
[9]. Vaideeswar P, Pandit SP, Primary zygomycosis of the pericardium in an immunocompetent hostMycoses 2007 50(3):232-34.10.1111/j.1439-0507.2006.01355.x17472623 [Google Scholar] [CrossRef] [PubMed]
[10]. Jain D, Kohli K, Neonatal gastrointestinal mucormycosis clinically mimicking necrotizing enterocolitisEur J Pediatr Surg 2009 19:405-07.10.1055/s-0029-120225119408216 [Google Scholar] [CrossRef] [PubMed]
[11]. Carr EJ, Scott P, Gradon JG, Fatal gastrointestinal mucormycosis that invaded the postoperative abdominal wound in an immunocompetent hostJ Clin Inf Dis 1999 29:956-57.10.1086/52048210589934 [Google Scholar] [CrossRef] [PubMed]
[12]. Sharma M, Gill SS, Kashyap S, Kataria R, Gupta D, Sahani P, Gastrointestinal mucormycosis an uncommon isolated MucormycosisIndian J Gastroenterol 1998 17(4):131-33. [Google Scholar]
[13]. Virk SS, Singh RP, Arora AS, Grewal JS, Puri H, Gastric zygomycosis – an unusual cause of massive upper gastrointestinal bleedIndian J Gastroenterol 2004 23(4):146-47. [Google Scholar]
[14]. Kontoyiannis DP, Lewis RE, “How I treat mucormycosis”Blood 2011 118(5):1216-24.10.1182/blood-2011-03-31643021622653 [Google Scholar] [CrossRef] [PubMed]
[15]. Shahpure AG, Patankar RV, Bhatkhande R, Gastric mucormycosisInd J Gastroenterol 2002 21:231-32. [Google Scholar]
[16]. Laura W, Lamp. Infectious disorders of the GI tract. In: Odze RD, Goldblum JR, editorsSurgical pathology of the GI tract, liver, biliary tract and pancreas 2009 2nd edPhiladelphiaElsevier Inc:51-79.10.1016/B978-141604059-0.50007-2 [Google Scholar] [CrossRef]
[17]. Roussy JF, Allard C, St-Germain G, Pépin J, Gastrointestinal mucormycosis following a streptococcus pyogenes toxic shock syndrome in a previously healthy patient: a case reportCase Rep Infect Dis 2012 2012:47671910.1155/2012/47671922900216 [Google Scholar] [CrossRef] [PubMed]
[18]. Spellberg B, Ibrahim AS, Chin-Hong PV, Kontoyiannia D, Morris M, Fredericks D, The Deferasirox-AmBisome therapy for mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trialJournal of Antimicrobial Chemotherapy 2012 673:715-22.10.1093/jac/dkr37521937481 [Google Scholar] [CrossRef] [PubMed]
[19]. Greenberg RN, Mullane K, Van Burik AH, Raad I, Abzug MJ, Anstead G, Posaconazole as salvage therapy for zygomycosisAntimicrobial Agents and Chemotherapy 2006 50:126-33.10.1128/AAC.50.1.126-133.200616377677 [Google Scholar] [CrossRef] [PubMed]
[20]. Ibrahim AS, Gebremariam T, Schwartz J, Edwards JE, Spellberg B, Posaconazole mono- or combination therapy for treatment of murine zygomycosisAntimicrobial agents and chemotherapy 2009 53(2):772-75.10.1128/AAC.01124-0818936190 [Google Scholar] [CrossRef] [PubMed]