Gall bladder carcinoma is an aggressive malignancy with varied presentation. Liver is most commonly involved initially with distant metastasis in virtually all possible organs. We report a case of middle aged female who presented with pain in upper abdomen and progressive jaundice. Imaging studies were suggestive of gall bladder carcinoma involving hilum with disseminated osteosclerotic lesions and metastasis to Urinary Bladder (UB). Patients of carcinoma gall bladder with disseminated metastatic disease have poor prognosis and are managed with palliative chemotherapy. To the best of our knowledge, this is one of the rare initial presentation of gall bladder carcinoma in the literature.
Disseminated, Jaundice, Osteosclerotic
Case Report
A 53-year-old female presented with complaints of pain in the right upper abdomen and yellowish discoloration of both eyes and urine for past three months. Her jaundice was associated with itching and clay coloured stools. She also grumbled about the generalized back ache and pain in bilateral chest wall. On examination, she was icteric and per abdominal examination revealed a palpable gall bladder lump apart from mild hepatomegaly. There was mild tenderness along the spine. No other significant finding or lymphadenopathy was noted.
Biochemical investigations revealed a raised serum bilirubin (14.8 mg%) and alkaline phosphatase level (3193 IU/L). An ultrasound examination of the abdomen reported an asymmetric thickening of the gall bladder wall in the body region extending on to its neck [Table/Fig-1a]. Bilateral intra hepatic biliary radicals were dilated with cut off at the level of hilum due to soft tissue thickening extending from gall bladder neck to primary biliary confluence. So, a provisional diagnosis of carcinoma gall bladder was made.
a) Grey scale ultrasound image shows gall bladder wall thickening with cholelithiasis; b) Contrast enhanced CT axial section confirming enhancing gall bladder wall thickening (red arrow) along with periportal, celiac lymphadenopathy.; c) Axial minimum intensity projection (MinIP) image shows bilobar intrahepatic biliary radical dilatation with mild perihepatic free fluid; d) Sagittal reformat shows multifocal enhancing urinary bladder wall thickening (red arrowhead) with ascites.
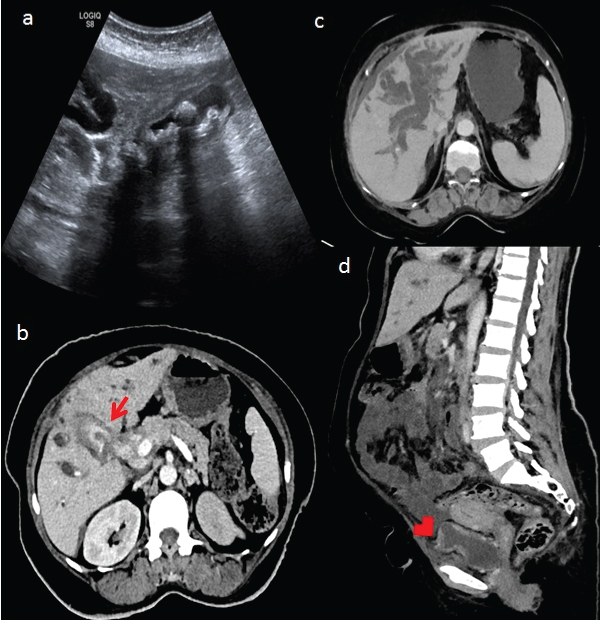
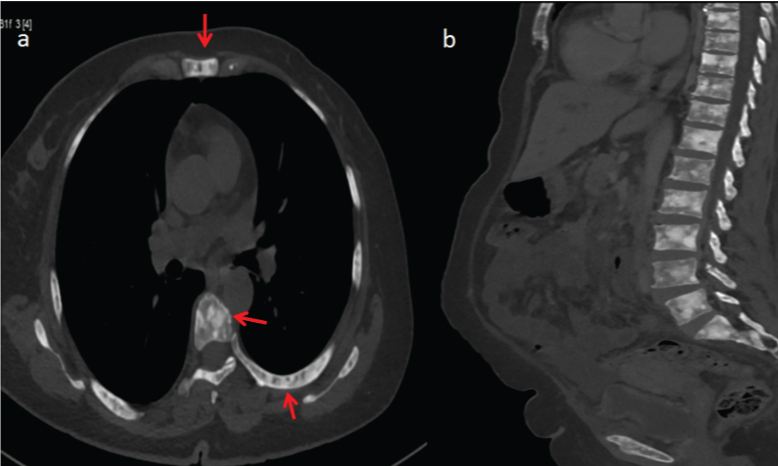
Her Carcinoembryonic Antigen (CEA) and CA 19-9 were >200 ng/mL and 97.36 U/mL respectively. Further evaluation with contrast enhanced CT (CECT) scan of the chest and abdomen confirmed the findings of carcinoma of the gall bladder neck extending into hilum with no significant liver infiltration [Table/Fig-1b,c]. Periportal lymphadenopathy was present along with mild ascites. Enhancing multifocal thickening of the UB wall was evident [Table/Fig-1d]. In addition, ribs, sternum and vertebral bodies were studded with diffuse sclerotic bone lesions [Table/Fig-2a,b]. There was no lung metastasis.
Axial and sagittal CT images show multiple sclerotic leions in sternum, ribs and vertebrae (arrows).
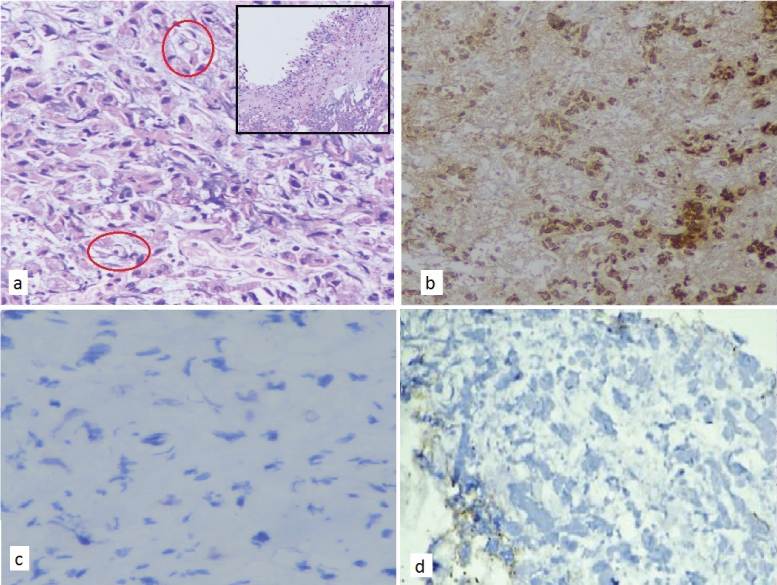
An ultrasound guided fine needle aspiration from the gall bladder thickening was attempted twice but yield was very poor. Urine culture and exfoliative cytology were negative. Despite, no history of haematuria, cystoscopy was performed to rule out malignancy in view of suspicious thickening but on gross examination, mucosa was unremarkable. However, random biopsies were taken from the bladder wall. Surprisingly, biopsy from UB was suggestive of adenocarcinoma with few signet ring cells [Table/Fig-3a]. The tumour cells showed intense cytoplasmic positivity for pan-cytokeratin [Table/Fig-3b] and mucin was highlighted by Mayer’s mucicarmine stain. Immunohistochemistry (IHC) markers were negative for urothelial and colonic origin [Table/Fig-3c,d] and suggestive of metastatic disease.
Urinary bladder biopsy; a) Diffuse infiltration by tumour cells, cords and vague acinar pattern on Haematoxylin and Eosin (H&E) (10X) with signet ring cells highlighted in red circle. Benign urothelial lining on H&E (inset); b) Immunohistochemistry (IHC) for Pan cytokeratin showing intense cytoplasmic positivity; c) and d) IHC for CDX2 and GATA 3 respectively were negative, ruling out both colorectal and urothelial origin.
Thus, a diagnosis of carcinoma of the gall bladder with obstructive jaundice associated with metastases to UB and osteosclerotic secondaries was made. In view of raised bilirubin with separated primary biliary confluence, percutaneous Transhepatic Biliary Drainage (PTBD) was performed. After PTBD, her bilirubin was on declining trend and she was planned for chemotherapy. However, she suffered an episode of cholangitis and despite antibiotic treatment, succumbed to cholangitis before starting chemotherapy after two months of diagnosis.
Discussion
Gall bladder cancer (GBC) is the most common malignancy of the biliary tract [1]. The common mode of presentation are pain in abdomen, anorexia, weight loss, obstructive jaundice and awareness of lump. Most of the patients have advanced disease at the time of diagnosis. GBC spreads rapidly and by all possible routes viz. local, lymphatic, haematogenous, neural, and transcoelomic. GBC usually involves the adjacent organs such as liver, bile duct, duodenum, and colon. The involvement of lymph nodes occur simultaneously involving hepato duodenal ligament, peri pancreatic and para aortic region [2].
Further, distant metastasis in GBC has been reported in practically all organs [3]. However, liver is involved primarily with metastatic disease and only one fifth of cases involve other sites [4]. Incidence of bony metastasis from GBC has been reported in ~ 10% in one autopsy series, which show that bone metastases may occur in advanced stages [4]. However, early bone metastasis may go unnoticed due to the presence of symptoms related to advanced local disease [2].
Silent bone metastasis in GBC have been reported in literature at the time of presentation. Osteolytic bone metastasis in isolation have been reported at femur, lumbar vertebrae, humerus, clavicle, scalp [2,4-6]. However, only one case report of osteosclerotic bony metastases was reported by Kumar A et al., [3]. Our case seems to be another one with disseminated osteosclerotic bony secondaries in GBC.
Transcoelomic spread usually leads to peritoneal, omental and mesenteric deposits or blummer’s shelf where tumour cells deposits in the pouch of douglas. Apart from this, krukenberg tumor is also reported in pre menopausal ladies. Metastatic adenocarcinoma in UB is a rare entity. The incidence of primary adenocarcinoma of UB is ~0.5-2% [7], however, metastatic adenocarcinomas in UB are more common than primary [8]. UB gets involved by either direct extension or via a haematogenous/lymphatic route. Secondary bladder adenocarcinoma commonly arises from colon, prostate, endometrium, cervix, breast and lung [7]. We also encountered metastases to UB from GBC in our case.
The histological features of adenocarcinoma with signet ring cells in UB suggest several differential diagnoses. It may be a primary adenocarcinoma, Plasmacytoid Urothelial Carcinoma (PUC), metastatic carcinoma especially from colon, breast, stomach, and plasma cellderived neoplasms including lymphomas and plasmacytomas. The IHC markers for primary adenocarcinoma of UB are GATA3 & CDX2, for PUC are CD 138 and P63, CD 45 for lymphoma and S-100 for melanoma [9]. All these IHC markers were negative in the index case ruling out urothelial origin of tumour.
In view of the patient demographics, middle aged lady with imaging studies suggestive of malignancy in gall bladder with bone metastasis, metastasis in UB was likely secondary to GBC. The possible explanation of this rare event is bone metastasis from gall bladder malignancy via haematogenous route. Later, from batson’s plexus which is a valve less communication between bone and UB, the malignant cells reached to UB.
Ureteric obstruction secondary to metastasis from GBC has been reported twice [10,11]. Similarly, coincidence of GBC with metastatic lesion in UB has also been stated in available reviewed literature [12,13]. In our case, we found two rare metastatic sites in patient of GBC. Till date, there have been no reports of GBC with concomitant disseminated osteosclerotic metastases to bone and to UB.
The management of patient with multifocal metastases is challenging. Biliary drainage is usually performed to relieve cholangitis and to bring down the level of bilirubin so that palliative chemotherapy can be offered. But, the outcomes following palliative chemotherapy are also not worthy [2]. The aim of treatment in patients with metastatic disease remains palliative. Mollifying treatment is given for relief of symptoms to improve the quality of life with minimum adverse effects. Oral analgesics like paracetamol, tramadol can be accompanied with analgesic patches like fentanyl for pain. Bisphosphonates show promising results in osteolytic bone diseases but appear to be less effective in osteosclerotic bone disease [14].
Conclusion
We suggest that patient with multifocal metastases should be enrolled in clinical trials like mutational analysis to find best management strategies for with multifocal metastases diseases. Keeping this in mind we conclude that though pelvic organ and bone are rare sites of gall bladder metastases but one should keep vigilance about rare sites of metastatic disease. Whether investigation for bony metastasis should be included in the routine work up protocol of GBC remains a question to be answered.
[1]. Carriaga MT, Henson DE, Liver, gallbladder, extrahepatic bile ducts, and pancreasCancer 1995 75(1 Suppl):171-90.10.1002/1097-0142(19950101)75:1+<171::AID-CNCR2820751306>3.0.CO;2-2 [Google Scholar] [CrossRef]
[2]. Joshi MK, Joshi R, Chadha M, Alam SE, Varshneya H, Kumar S, Gall Bladder Carcinoma Presenting with Spinal Metastasis: A Rare PhenomenonIndian J Palliat Care 2013 19(2):113-15.10.4103/0973-1075.11671124049354 [Google Scholar] [CrossRef] [PubMed]
[3]. Kumar A, Bhargava SK, Upreti L, Kumar J, Disseminated osteosclerotic skeletal metastasis from carcinoma gall bladder: a case reportIndian J Radiol Imaging 2003 13(1):37-39.10.4103/0971-3026.5975020351990 [Google Scholar] [CrossRef] [PubMed]
[4]. Singh S, Bhojwani R, Singh S, Bhatnagar A, Saran RK, Agarwal AK, Skeletal metastasis in gallbladder cancerHPB (Oxford) 2007 9(1):71-72.10.1080/1365182060111007118333116 [Google Scholar] [CrossRef] [PubMed]
[5]. Kohli A, Chib RS, Parihar BK, Secondary involvement of femur in carcinoma of gall bladderIndian J Cancer 1994 31(4):257-59. [Google Scholar]
[6]. Pandey M, Aryya NC, Pradhan S, Asthana AK, Gautam A, Shukla VK, Carcinoma of the gallbladder presenting as scalp tumourEur J Surg Oncol 1998 24(6):605-07.10.1016/S0748-7983(98)93968-3 [Google Scholar] [CrossRef]
[7]. Roy S, Smith MA, Cieply KM, Acquafondata MB, Parwani AV, Primary bladder adenocarcinoma versus metastatic colorectal adenocarcinoma: a persisting diagnostic challengeDiagn Pathol 2012 7:15110.1186/1746-1596-7-15123121893 [Google Scholar] [CrossRef] [PubMed]
[8]. Bates AW, Baithun SI, Secondary neoplasms of the bladder are histological mimics of nontransitional cell primary tumours: clinico-pathological and histological features of 282 casesHistopathology 2000 36(1):32-40.10.1046/j.1365-2559.2000.00797.x10632749 [Google Scholar] [CrossRef] [PubMed]
[9]. Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE, The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder TumoursEur Urol 2016 70(1):106-19.10.1016/j.eururo.2016.02.02826996659 [Google Scholar] [CrossRef] [PubMed]
[10]. Claire RJ, Cohen EL, Rudick DH, Adenocarcinoma of gallbladder producing unilateral ureteral obstructionUrology 1979 14(2):163-66.10.1016/0090-4295(79)90151-1 [Google Scholar] [CrossRef]
[11]. Sarma D, Hanemann M, Adenocarcinoma of gallbladder presenting as ureteral obstructionUrology 1985 25(1):60-62.10.1016/0090-4295(85)90566-7 [Google Scholar] [CrossRef]
[12]. Jindal T, Mandal SN, Kamal MR, Das AK, Karmakar D, Occult mucin secreting adenocarcinoma of gall bladder with metastasis to urinary bladderUpdates Surg 2012 64(3):239-40.10.1007/s13304-011-0094-321720916 [Google Scholar] [CrossRef] [PubMed]
[13]. Brock JE, Ahmed A, Lofts FJ, Adenocarcinoma of the gall bladder presenting with haematuriaClin Oncol (R CollRadiol) 2001 13(1):65-66. [Google Scholar]
[14]. Suva LJ, Washam C, Nicholas RW, Griffin RJ, Bone metastasis: mechanisms and therapeutic opportunities Nat Rev Endocrinol 2011 7(4):208-18.10.1038/nrendo.2010.22721200394 [Google Scholar] [CrossRef] [PubMed]