Cutaneous metastasis is a relatively rare complication of internal malignancies with a reported incidence of 0.7-9%. Ten percent of the patients with metastatic cancer have cutaneous metastases [1,2]. Clinically, cutaneous metastases can sometimes mimic common dermatoses like cellulitis, epidermal inclusion cysts, ulcers; thus making the diagnosis difficult [3]. The most common sources of cutaneous metastases are breast, lung, colon, stomach, upper aerodigestive tract, uterus and kidney; the most common skin metastases from a previously unknown primary tumour originate from the kidney, lung, thyroid or ovary [4-6]. Cutaneous metastases are usually indicative of disseminated disease and indicate a correspondingly poor prognosis; survival is typically only about three months in patients with disseminated skin metastases [7]. Their recognition is important as they can be the first sign of extranodal metastatic disease and hence, can have profound prognostic implications. This study was conducted to understand the relative proportion of different internal malignancies metastasising to the skin and study the clinicopathological features in a tertiary care hospital in India.
Materials and Methods
This was a five year retrospective study that analysed patients with a proven diagnosis of metastatic carcinoma to skin on histopathology, conducted in the Department of Pathology, after obtaining Institutional Ethical Committee clearance during the period January 2010 to December 2014. Eighteen cases with a diagnosis of metastastic carcinoma to skin were retrieved from Pathology database in the Department of Pathology. Only cases with a diagnosis of solid internal malignancy recorded consecutively from 2010 to 2014 in the archives were included, and cases of haematolymphoid neoplasms were excluded from the study. Also, cases with direct extension of primary malignancy into the overlying skin were excluded. Information pertaining to patient demographics, duration of skin lesions, clinical presentation and site of lesions, details of previous internal malignancy, response to treatment and prognosis were noted from patients medical records and histopathological features were obtained from pathology reports.
Results
Eighteen patients with cutaneous metastasis were identified. There were 10 males (55.56%) and eight females (44.44%). Mean age was 52.33 years (range 30 to 73 years).
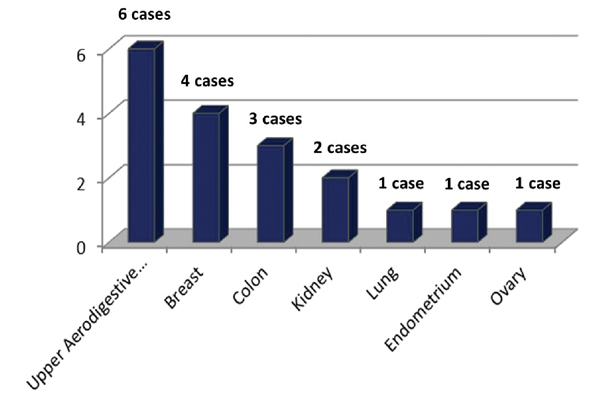
In 12 (66.67%) patients, cutaneous metastases appeared in patients with history of known malignancy after a mean duration of 25.75 months (range two months-20 years). Cutaneous metastasis was the presenting feature in six (33.33%) patients. The most common internal malignancy was from upper aerodigestive tract, followed by breast as shown in [Table/Fig-1].
Site of primary malignancy.
The lesions were multiple in all cases and the clinical presentation was painless, firm to hard cutaneous nodules in 10 (55.56%) patients, ulcerated nodules in six (33.33%), painful nodules in one (5.56%) patient and indurated plaques in one (5.56%) patient [Table/Fig-2].
Clinicopathological data of patients with cutaneous metastasis.
| S. No. | Age (years) and Sex (M/F) | Primary malignancy | Time interval* | Clinical presentation | Site | Type | Outcome, following diagnosis of cutaneous metastasis |
|---|
| 1. | 40, F | Infiltrating duct carcinoma of breast | Eight months | Painless nodules | Chest, scar back | Contiguous | Lost to follow up |
| 2. | 42, M | Adenocarcinoma of colon | 22 months | Ulcerated nodules | Abdominal wall | Contiguous | Succumbed within one month |
| 3. | 50, F | Squamous cell carcinoma of tongue | Four years | Ulcerated nodules | Face | Contiguous | Lost to follow up |
| 4. | 58, M | Squamous cell carcinoma of tongue | Two years | Painless nodules | Neck | Non-contiguous | Lost to follow up |
| 5. | 55, F | Adenocarcinoma of endometrium | Two months | Ulcerated nodules | Abdominal wall | Contiguous | Lost to follow up |
| 6. | 73, F | Adenocarcinoma of colon | Presenting feature | Painless nodules | Abdominal wall | Contiguous | Improved with treatment |
| 7. | 49, M | Squamous cell carcinoma of soft palate | Four months | Painless nodules | Neck shoulder | Non-contiguous | Improved with treatment |
| 8. | 70, M | Adenocarcinoma of colon | Presenting feature | Painless nodules | Abdominal wall, scar | Contiguous | Improved with treatment |
| 9. | 58, M | Renal cell carcinoma | Presenting feature | Ulcerated nodules | Groins | Non-contiguous | Improved with treatment |
| 10. | 30, M | Adenocarcinoma of lung | Presenting feature | Painless nodules | Abdominal wall | Non-contiguous | Lost to follow up |
| 11. | 47, F | Infiltrating ductal carcinoma of breast | Two years | Painless nodules | Chest, scar thighs | Contiguous and Non-contiguous | Lost to follow up |
| 12. | 69, F | Serous cystadenocarcinoma of ovary | Presenting feature | Indurated plaques [Table/Fig-3] | Abdominal wall | Contiguous | Improved with treatment |
| 13. | 41, M | Renal cell carcinoma | Two years | Painful nodules | Abdominal wall, back | Contiguous and Non-contiguous | Lost to follow up |
| 14. | 52, M | Leiomyosarcoma alveolus and hard palate | 20 months | Painless nodules | Abdominal wall axilla groins | Non-contiguous | Lost to follow up |
| 15. | 61, M | Squamous cell carcinoma of tongue | Three months | Ulcerated nodules [Table/Fig-4] | Chest, scalp | Non-contiguous | Succumbed within one month |
| 16. | 50, F | Infiltrating duct carcinoma of breast | Presenting feature | Painless nodules [Table/Fig-5] | Abdominal wall, chest axilla | Non-contiguous | Succumbed after 16 months |
| 17. | 43, F | Infiltrating duct carcinoma of breast | 20 years | Painless nodules | Chest | Contiguous | Lost to follow up |
| 18. | 54, M | Squamous cell carcinoma of pharynx | Eight months | Ulcerated nodules | Neck | Non-contiguous | Improved with treatment |
*Time interval between diagnosis of primary malignancy and development of cutaneous metastases
M=Male, F=Female
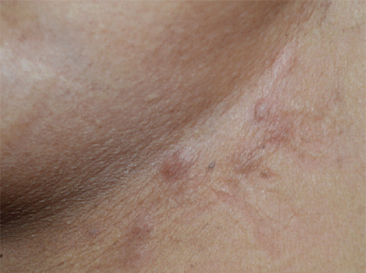
Indurated lesions over the thigh skin in a patient with serous cystadenocarcinoma of ovary.
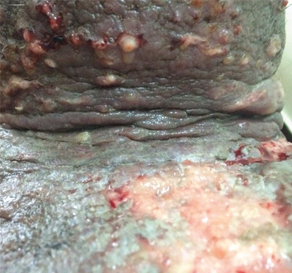
Ulcerated lesions on the neck in a patient with squamous cell carcinoma of tongue.
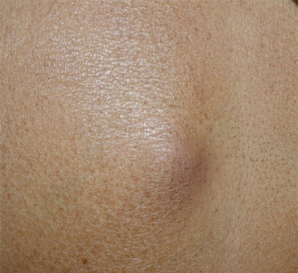
Nodular lesions over the abdominal wall in a patient with carcinoma breast.
Cutaneous metastases were associated with fatal outcome in three (16.67%) patients. Two patients, one with adenocarcinoma colon and other with carcinoma tongue succumbed in about a month after developing cutaneous metastasis. Another patient with carcinoma breast died 16 months after being diagnosed with cutaneous metastasis. Six patients improved following treatment. Nine patients were lost to follow up.
Discussion
The single most basic biologic process that characterises a malignant tumour is the ability to produce secondary deposits (metastases) at distant sites [8]. Cutaneous metastasis is defined as the spread of malignant cells from a primary malignancy to the skin. This may occur from spread of tumour cells from an internal malignancy or from a primary skin cancer [9]. Skin is not a common site for distant metastasis, and is reported to be involved in about 0.7-9% of all patients with cancer [1,2].
The most common internal malignancies giving rise to cutaneous metastases are lung and colon cancers in males and breast, colon and ovary cancers in females [2,10]. In our study, the most common internal malignancies with skin metastasis were breast carcinoma in females and colon, kidney and tongue cancers in males. In a study from Taiwan, the highest rates of skin metastases were found to occur from carcinoma of the breast, followed by the lung, oral mucosa, colon rectum, stomach and oesophagus [11].
Cutaneous metastases are generally detected during the advanced stages of malignancy, they can occasionally be the presenting feature of an internal malignancy [4]. Cutaneous metastases is reported as initial manifestation of underlying cancer in 34% patients in a study [12]. Most common primary site of cutaneous metastasis in females was reported from breast carcinoma followed by carcinoma of large intestine, lungs and ovaries, while in males in decreasing order were from lungs, large intestine, oral cavity, kidney and stomach [13]. Brownstein MH and Helwing EB, reported that lung, kidney and ovary cancers are the most common type of cancers with skin involvement as the presenting sign [10]. In our study, cutaneous metastasis was the presenting feature in 33.33% patients. Cancers of the breast, ovary, kidney, lung, colon and palate showed skin involvement as the presenting sign in our study. On the other hand, one patient with carcinoma breast developed cutaneous metastasis 20 years after being diagnosed with the breast cancer. Others developed skin metastatic deposits after a period ranging from two months to four years after being diagnosed with primary malignancy.
Clinically, cutaneous metastases have a varied presentation. The lesions may present as dermal papules, subcutaneous nodules, inflammatory patches, fixed, indurated lesions, ulcerated lesions or rarely as bullous lesions, cutaneous cysts, alopecia neoplastica, sclerodermoid or zosteriform lesions. The most common presentation is as multiple nodular lesions [2,9,14]. In our study, the most common presentation was multiple painless nodules in 10 patients followed by ulcerated lesions in six patients. One of our patients presented with painful nodules (renal cell carcinoma) and another patient with ovarian malignancy presented with diffuse cutaneous infiltration.
Cutaneous metastases show predilection for certain regions. Breast and lung cancers frequently metastasise to the chest wall, whereas cancers of the bowel, ovary, and bladder most often metastasise to the abdomen [12]. In our study, cutaneous metastasis was seen over the anterior chest wall in five patients, four of whom had breast cancer. Nine patients had abdominal wall metastasis, three of whom had bowel cancer and one each had ovarian cancer, endometrial cancer and renal carcinoma. Cutaneous metastasis was seen at the site of previous surgery scar in two patients with carcinoma breast and one patient with adenocarcinoma colon. Metastatic deposits over the skin were seen at contiguous sites in eight patients and non-contiguous sites in eight patients. Two patients showed skin metastases at both contiguous as well as non-contiguous sites.
Generally, cutaneous metastases herald a poor prognosis. They are usually indicative of disseminated, progressive disease or rarely recurrence of the primary tumour. The average survival time of patients with skin metastases is a few months [14]. The patients survived for an average of three months in one study [7]. In our study, two patients, one with adenocarcinoma colon and another with squamous cell carcinoma tongue succumbed to their illness in a month after being diagnosed with cutaneous metastasis. Two patients with carcinoma breast had a survival time of 10 and 16 months after developing cutaneous metatstatic deposits. On follow up, one patient with adenocarcinoma lung was surviving after a period of four years and a patient with carcinoma breast was surviving at seven years follow up.
Conclusion
Cutaneous metastases are uncommon but early recognition of cutaneous metastasis is very important as they may be the first presentation of an internal malignancy. Cutaneous metastases are highly variable in presentation and a high-index of suspicion, especially in patients with a history of cancer, is a must for prompt diagnosis. Only with prompt recognition comes the opportunity to treat the systemic spread of the cancer in early stage and thus improve the prognosis. Inspite of a low-risk of cutaneous metastasis, skin nodules of an unknown cause should be biopsied to rule out cutaneous metastasis.
*Time interval between diagnosis of primary malignancy and development of cutaneous metastases
M=Male, F=Female