Outcome of Critical Pregnant and Postpartum Patients of Swine flu- Experience of Seven Years
Archana Mishra1, Harish Chandra Sachdeva2, Sunita Malik3, Archana Kumari4
1 Associate Professor, Department of Obstetrics and Gynaecology, Vardhman Mahavir Medical College and Safdarjung Hospital, Delhi, India.
2 Professor, Department of Anaesthesia, Vardhman Mahavir Medical College and Safdarjung Hospital, Delhi, India.
3 Professor, Department of Obstetrics and Gynaecology, Vardhman Mahavir Medical College and Safdarjung Hospital, Delhi, India.
4 Postgraduate Student, Department of Obstetrics and Gynaecology, Vardhman Mahavir Medical College and Safdarjung Hospital, Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Archana Mishra, House No. 26-A, Pocket 4, Mayur Vihar Phase-1, East Delhi-110091, India.
E-mail: drarchanamishra@rediffmail.com
Introduction
Pregnancy and postpartum confers four to five fold increased risk for complications and mortality if affected by Swine flu. Swine flu in pregnancy contributes to almost 18-25% of total ICU admissions and high perinatal morbidity and mortality.
Aim
The present study was an analysis of the pattern of critical illness and mortality due to Swine flu virus in pregnant and postpartum women.
Materials and Methods
It was a retrospective study of all clinically suspected, probable as well as confirmed cases of Swine flu, in pregnancy and postpartum period up to two weeks; who were admitted to Swine flu Intensive Care Unit (ICU) from February 2009 to December 2015. We analyzed the presenting complaints, condition on admission, criteria for admission in ICU, abnormalities in laboratory reports, course of illness and perinatal outcome.
Results
In the span of seven years 30 pregnant and 10 postpartum women were admitted in Swine flu ICU. Mortality was 40% (16 out of 40) in pregnant and postpartum women. There were 17 (42.5%) cases which were positive for H1N1 confirmed by Real time-PCR (RT-PCR) and culture. Mean interval of onset of symptoms to start of oseltamivir was four and a half days. Perinatal outcome was poor and only 18% babies were alive after one week of birth.
Conclusion
We emphasize that high index of suspicion, early diagnosis, early antiviral therapy and immunization to pregnant women are the key factors which can reduce the complications, ICU admissions and mortality in this group.
Influenza, Perinatal outcome, Postpartum period, Pregnancy
Introduction
In April 2009, cases of a new type of influenza [H1N1] were diagnosed in Mexico and United States which spread rapidly to other continents [1]. In 2009, World Health Organization (WHO) declared pandemic of Swine Flu which involved 206 countries, over 600,000 individuals and resulted in 6,250 deaths [2]. Swine flu [H1N1] is the triple reassortant virus which has genes of Avian, Swine and Human influenza [3]. In 2009 pandemic illness ranged from mild to severe. According to an analysis by the Centre for disease control 2009 H1N1 flu caused greater disease burden in young people than older people [4]. Pregnant and recently delivered women are at high risk as they suffer four to five fold increase in complications of Swine flu [5]. In 2009, pandemic 18-25% of patients with swine flu admitted in ICU involved pregnant and recently delivered women [6]. Pneumonia is more commonly seen in pregnant women if affected by Swine flu due to the immune suppressed status, reduced tidal volume, localized oedema and congestion of lungs [3]. Swine Flu has also been associated with high perinatal mortality and morbidity [7]. Foetal hypoxia is usually associated with high fever and Adult Respiratory Distress Syndrome (ARDS) in mother. India was amongst those countries affected by the 2009 pandemic of Swine flu. After that there were outbreaks in 2013 and 2015 and small number of cases was reported in between. Aim of the present study was to analyze the maternal and perinatal outcome of pregnant and postpartum patients with Swine flu requiring intensive care. Significance of our study lies in the analysis of determinants for severe disease and mortality in this vulnerable group. Knowledge of these factors can be utilized for prevention and appropriate management of Swine flu in pregnancy.
Materials and Methods
This was a retrospective cohort study of all clinically suspected, probable as well as confirmed cases of Swine flu in pregnancy and postpartum admitted to Swine flu ICU from February 2009 to December 2015. Institutional Ethical clearance was taken before performing the study from Vardhman Mahavir Medical College and Safdarjung Hospital, Delhi, India. In the present study, we included women up to two weeks postpartum because after that risks equal to the general population [8,9]. Evaluation of all the clinically suspected cases was done because many patients died before laboratory confirmation. Moreover, sensitivity of RT-PCR is only 10% to 65% depending upon the conditions of sampling and transportation [10,11]. All clinically suspected critical case of Swine flu in pregnancy and/or up to two weeks postpartum who were admitted in ICU were included in the study. Symptoms which were taken in account were fever, cough, sore throat, rhinorrhoea, muscle pain, headache, chills, malaise, diarrhoea, vomiting along with the features of severe disease. Severe features were chest pain, poor oxygenation (e.g., tachypnea, hypoxia), haemodynamic instability, CNS impairment and severe dehydration. Laboratory confirmation by RT-PCR and virus isolation was also taken in account. Cases where any other cause was diagnosed by further evaluation were excluded from the study. Records of all these cases were thoroughly evaluated. Demographic profiles of all the cases were evaluated along with the obstetric histories. Any other medical condition complicating pregnancy was also documented. Patient’s presenting complaints were assessed as well as the treatment taken before reaching our hospital was recorded. Time interval between onset of symptoms and starting antiviral therapy was documented. Patient’s vital signs on admission, laboratory and radiographic result and criteria for admission in ICU were noted down. Intrapartum complications such as antepartum haemorrhage and postpartum haemorrhage were noted. Perinatal outcome was also recorded. Determinants of severe disease were assessed. Data is presented in percentage as well as numbers.
Results
A total of 40 patients with Swine flu in pregnancy and postpartum were admitted to the ICU over the period of nearly seven years from February 2009 to December 2015. [Table/Fig-1] showed annual admissions and mortality of these patients during this period. Mean age of patients was 27.2 years. Primigravida constituted 10 (33.3%) of total 30 pregnant women. Rest were multigravida (n=20). Mean gestational age at the time of admission was 32.6 weeks. Most cases were low risk and comorbidities were present in 11 cases.
Admissions and mortality in dedicated Swine flu ICU.
x-axis denotes the year and y-axis denotes the number of patients
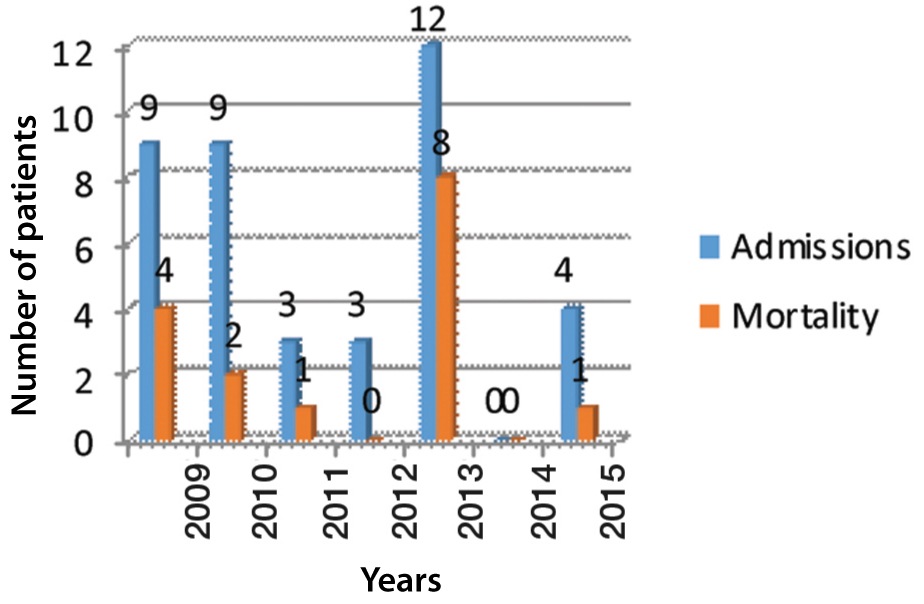
At the time of admission 30 women were pregnant and 10 women were postpartum. None of the women were in first trimester of pregnancy. Out of pregnant women 9 (30%) were in second trimester and 21 (70%) in third trimester. In these 40 patients, 17 were H1N1 positive confirmed by RT-PCR followed by viral culture. Laboratory confirmation was 17 out of 40 (42.5%) for Swine flu. Mean interval of starting antiviral therapy (oseltamivir) was four and half days (range two to 10 days) after the onset of the symptoms. Only one patient received antiviral therapy within 48 hours of onset of the symptoms. The longest interval was 10 days in a patient who was referred from another state. Overall mortality in these patients was 16 out of 40 (40%). Anaemia was present in 7 (17.5%), hypertensive disorders of pregnancy was present in 3 (7.5%), past history of tuberculosis in 7 (17.5%) and pre-existing heart disease was present in 4 (10 %) of patients. None of the patients had history of asthma, smoking, or vaccination against influenza. None of our patients received oseltamivir before admission. Most of the cases were referred from another medical facility. All the patients were treated with acetaminophen before admission. Other medicines which they used were antibiotics and cold remedies. Presenting complaints of the patients are listed in [Table/Fig-2].
Presenting symptoms of the patients.
| S.No. | Symptoms | Number | Percentage(%) |
|---|
| 1 | Fever | 40 | 100 |
| 2 | Cough | 34 | 85 |
| 3 | Dyspnoea | 30 | 75 |
| 4 | Myalgia | 14 | 35 |
| 5 | Sore throat | 7 | 17.5 |
| 6 | Rhinorrhoea | 7 | 17.5 |
| 7 | Weakness | 4 | 10 |
| 8 | Chills | 7 | 17.5 |
| 9 | Decreased urine output | 7 | 17.5 |
| 10 | Oedema | 4 | 10 |
| 11 | Cyanosis | 7 | 17.5 |
| 12 | Labour pains | 1 | 2.5 |
None of our patient’s presented with nausea, vomiting, diarrhoea and dehydration. The condition at the time of admission is described in [Table/Fig-3] which shows that all the patients presented with fever above 100°F and tachycardia.
Condition at the time of admission.
| S.No. | Symptoms | Number | Percentage(%) |
|---|
| 1 | Fever above 100°F | 40 | 100 |
| 2 | Tachycardia | 40 | 100 |
| 3 | Hypotension | 14 | 35 |
| 4 | Hypertension | 4 | 10 |
| 5 | Crepitation / Rhonchi on auscultation | 17 | 42.5 |
| 6 | Altered Sensorium | 8 | 20 |
Pulmonary complications were the most common causes for need of admission to ICU followed by haemodynamic instability [Table/Fig-4]. [Table/Fig-5] is showing the abnormal laboratory results of these patients.
Reasons for Intensive care admission.
| Criteria of Severity | Number | Percentage(%) |
|---|
| Pneumonia | 17 | 42.5 |
| Intubation | 30 | 75 |
| ARDS | 34 | 85 |
| Pulmonary Oedema | 7 | 17.5 |
| Haemodynamic Instability | 14 | 35 |
ARDS = Adult Respiratory Distress Syndrome
Laboratory abnormalities.
| Laboratory abnormality | Number | Percentage(%) |
|---|
| Anaemia | 14 | 35 |
| Raised C-Reactive Protein | 30 | 75 |
| Raised LDH | 10 | 25 |
| Electrolyte imbalance | 7 | 17.5 |
| Renal failure | 14 | 35 |
| Raised liver enzymes | 14 | 35 |
| Thrombocytopenia | 7 | 17.5 |
| Raised CPK /CPK-MB | 7 | 17.5 |
| Leucocytosis | 4 | 10 |
| Leucopenia | 4 | 10 |
| Consolidation on chest X-ray | 17 | 42.5 |
| Decreased Oxygen saturation on ABG | 30 | 75 |
| Coagulation failure | 7 | 17.5 |
ABG -Arterial blood gas analysis, CPK – Creatine Phosphokinase, CPK-MB- Creatine Phosphokinase isoenzyme MB), LDH-Lactate Dehydrogenase
Blood culture was negative in all these cases. All of the patients were treated with antiviral therapy oseltamivir 75 mg twice daily along with oxygen therapy without waiting for laboratory results. Non-invasive ventilation was used in 10 (25%) patients and rest required mechanical ventilation. Beta agonist was used for nebulization in 16 (40%) patients. Intravenous antibiotics were administered to 32 (80%) women. Corticosteroids, diuretics and other supportive treatment were administered as per need. During their stay in ICU, 11 women had delivery. Maternal outcome in relation to delivery is described in [Table/Fig-6].
Outcome of 30 pregnant patients in relation to delivery.
| Maternal Outcome | Number | Percentage(%) |
|---|
| Died undelivered | 9 | 30 |
| Died after delivery | 5 | 16.6 |
| Discharged undelivered | 10 | 33.3 |
| Discharged after delivery | 6 | 20 |
Known pregnancy outcome (n=11): stillbirths 6 (54.5%), miscarriage 2 (18.2%), neonatal deaths 1 (9%) and alive babies were 2 (18.2%).
The mode of delivery of these 11 women were as follows. Vaginal delivery after induction of labor occurred in six women and three women had an instrumental delivery for prevention of maternal exhaustion in second stage. Two women had caesarean section for obstetric indications. Out of 10 women who were admitted in postpartum two died and rest discharged in satisfactory condition. Out of 17 laboratory confirmed cases 16 were pregnant and one was postpartum. Outcome of laboratory confirmed cases it was as follows. Patients who died before delivery were seven. Four pregnant patients were discharged in satisfactory condition. Two patients died after delivery and two patients were discharged after delivery. Postpartum woman with laboratory confirmation was discharged in satisfactory condition.
Discussion
Indian National Data on Swine flu revealed that India was affected by 2009 pandemic [12]. In 2009, a total of 27,236 cases were reported from India, with mortality of 981 cases. In 2015, another outbreak was noticed with 8423 cases but a decreased death rate (585 deaths) [13]. This may be attributed to better preparedness in terms of awareness both in public and medical personnel, early testing and administration of oseltamivir at peripheral health facilities. Early testing has lead to early referral to specialised centres and better management so ICU admissions and deaths were much lower than previous years. However, another possibility is that the specific Virus type may be less virulent. There were very few studies on ICU admitted women who were very recently pregnant or still pregnant and suffered from Swine flu. The strength of the present study was that the pattern of disease was observed for almost seven years. To the best of our knowledge and search of literature it is only study of such long duration about critically ill pregnant and postpartum patients with Swine flu. In our study, we did not exclude the patients in lack of laboratory confirmation of RT–PCR because according to WHO “Laboratory confirmation of influenza virus infection is not necessary for the initiation of treatment and a negative laboratory test for H1N1 does not exclude the diagnosis in all patients” [10]. Although, it has been suggested that both seasonal influenza and pandemics are more serious in pregnancy [14]. We found that critical illness and death in sporadic cases is much less. Possible explanation is that in outbreaks of Swine flu, virus becomes more virulent. We found that advanced pregnancy and delay in starting antiviral therapy were the only two important determinants which correlated well with complications and mortality in critically ill patients of Swine flu [15]. Another study published from our own institute supports our observation [16]. That study included 24 H1N1 confirmed pregnant patients irrespective of severity of illness from January 2013 to March 2013. ICU admission was 5 (20.8%) and mortality was 3 (12.5%). All the deaths occurred in third trimester only. That study and present study share five patients in common. Our observation is supported by another study which documented five out of six deaths in such patients, occurred in third trimester [17]. One Indian study from 2009 pandemic supports the similar observation where mortality was 80% in third trimester as compared to 63% in early pregnancy [18]. Few other studies had shown large proportions of ICU admissions and mortality in advanced pregnancy and that too if there is delay in starting antiviral therapy [19,20]. Mortality in present study was 40% which is much higher than the other studies from Australia and New Zealand which reported a mortality of 11% in pregnant and postpartum patients [21]. Even one study from North India which was performed on Influenza like illness on 266 pregnant women has reported mortality of only 8% but all the deaths occurred in H1N1 positive patients that too in third trimester [22]. Another study from Brazil reported no death as all the patients received antiviral therapy and specialised care within 24 hours of symptoms [23]. Higher mortality in present study may be attributed to the particular cohort of critically ill patients and delay in starting antiviral therapy. In present study, patients reported at four to seven days of onset of symptoms after being treated at peripheral centres [mean interval four and a half days]. Another Study conducted by Pramanick A et al., reported median reporting time of non-survivor pregnant women suffering from influenza as six days as compared to one and a half days in survivors [24]. Third factor is lack of vaccination against influenza in pregnant women in our country. Studies from North India revealed less awareness in pregnant women as well as medical personnel regarding vaccination against Influenza [25]. Studies performed on vaccinated pregnant women had reported 36% reduction in rate of febrile respiratory illness and 63% reduction in rate of laboratory confirmed influenza in immunized pregnant women [26]. Results of present study are neither in favour nor in against the termination of pregnancy in hope of improvement. It seems wise to deliver a near-term pregnant patient suffering from ARDS by caesarean section. This approach is supposed to help in mechanical ventilation by improving chest compliance and respiratory performance in absence of mechanical effects of the enlarged uterus on the diaphragm and the respiratory system. In fact, some studies reported the caesarean rate as high as 50% in such patients [23]. We were unable to find definitive evidence of improvement after caesarean section. Critically ill patients of the present study had multi-organ involvement like thrombocytopenia and coagulopathies. We suggested these issues to be taken care of and patient should be stabilized before termination of pregnancy as labor and operative interventions poses additional stress on already decompensated systems. We recommend decision of termination and mode of termination should be judiciously taken by a senior clinician on case to case basis.
Limitation
Constraint of the present study was that it was a retrospective study. Complete data of seven years of all the patients of Swine flu especially who were treated in outpatient basis is lacking. We recommend higher quality prospective study for quantification of disease burden and benefit of early antiviral therapy. Similarly, safety and efficacy of universal immunization of pregnant women against influenza is an area of research.
Conclusion
We recommend national level policies for increasing awareness, acceptance and availability of influenza vaccine for medical personnel as well as pregnant women. There should be surveillance for seasonal influenza and early antiviral therapy and referral to specialised care for pregnant patients of influenza.
ARDS = Adult Respiratory Distress Syndrome
ABG -Arterial blood gas analysis, CPK – Creatine Phosphokinase, CPK-MB- Creatine Phosphokinase isoenzyme MB), LDH-Lactate Dehydrogenase
[1]. World Health Organization. World now at the start of 2009 influenza pandemic. Available from: http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html [Google Scholar]
[2]. World Health Organisation W. Pandemic (H1N1) 2009-update 76, Available from: http://www.who.int/csr/don/2009_11_27a/en/index.html; 2009 [Google Scholar]
[3]. Novel Swine-Origin Influenza A (H1N1) Virus Investigation TeamEmergence of a novel swine-origin influenza A (H1N1) virus in humansN Engl J Med 2009 360(25):2605-15.10.1056/NEJMoa090381019423869 [Google Scholar] [CrossRef] [PubMed]
[4]. Swine influenza A (H1N1) infection in two children—Southern California, March-April 2009. Morbidity and Mortality Weekly Report. Centers for Disease Control. 22 April 2009. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5815a5.htm [Google Scholar]
[5]. Centers for Disease Control and PreventionHospitalized patients with novel influenza A (H1N1) virus infection California, April-May 2009MMWR Morb Mortal Wkly Rep 2009 58:470 [Google Scholar]
[6]. Singanayagam A, Wood V, Chalmers JD, Factors associated with severe illness in pandemic 2009 influenza a (H1N1) infection: Implications for triage in primary and secondary careJ Infec 2011 63(4):243-51.10.1016/j.jinf.2011.07.01421839111 [Google Scholar] [CrossRef] [PubMed]
[7]. Beigi RH, Hodges J, Baldisseri M, English D, Magee-Women’s Hospital Ethics CommitteeClinical review: considerations for the triage of maternity care during an influenza pandemic one institution’s approachCrit Care 2010 14:22510.1186/cc892820587086 [Google Scholar] [CrossRef] [PubMed]
[8]. Centers for Disease Control and Prevention. Update Interim Recommendations for Obstetric Health Care Providers Related to Use of Antiviral Medications in the Treatment and Prevention of Influenza for the 2009–2010 Season. Available from: http://www.cdc.gov/h1n1flu/pregnancy/antiviral_messages.htm. Published 2009 [Google Scholar]
[9]. Figueiró-Filho EA, Obstetric, clinical, and perinatal implications of H1N1 viral infection during pregnancyIntl J Gynecol Obstet 2012 116:214-18.10.1016/j.ijgo.2011.10.02622196994 [Google Scholar] [CrossRef] [PubMed]
[10]. WHO information for laboratory diagnosis of pandemic (H1N1) 2009 virus in human revised. Available from: http://www.who.int/csr/resources/publications/swineflu/realtimeptpcr/en/index.html [Google Scholar]
[11]. http://www.who.int/csr/resources/publications/swineflu/storage_transport/en/index.html [Google Scholar]
[12]. Gandhi AB, Chhabra P, Arya S, Simmerman JM, Influenza and Pregnancy: A Review of the Literature from IndiaInfectious Diseases in Obstetrics and Gynaecology 2015 2015Article ID 867587, 810.1155/2015/86758725810687 [Google Scholar] [CrossRef] [PubMed]
[13]. http://www.mohfw.nic.in/showfile.php?lid=2121 [Google Scholar]
[14]. Dodds L, McNeil SA, Fell DB, Allen VM, Coombs A, Scott J, Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant womenCMAJ 2007 176(4):463-68.10.1503/cmaj.06143517296958 [Google Scholar] [CrossRef] [PubMed]
[15]. Tanaka T, Nakajima K, Murashima A, Garcia-Bournissen F, Koren G, Ito S, Safety of neuraminidase inhibitors against novel influenza A (H1N1) in pregnant and breastfeeding womenCMAJ 2009 181(1-2):55-58.10.1503/cmaj.09086619528139 [Google Scholar] [CrossRef] [PubMed]
[16]. Singhal S, Sarda N, Arora R, Punia N, Jain A, Clinical profile & outcome of H1N1 infected pregnant women in a tertiary care teaching hospital of northern IndiaIndian J Med Res 2014 139(3):454-58. [Google Scholar]
[17]. Jamieson D, Honein M, Rasmussen S, Williams J, Swerdlow D, Biggerstaff M, H1N1 2009 influenza virus infection during pregnancy in the USALancet 2009 374(9688):451-58.10.1016/S0140-6736(09)61304-0 [Google Scholar] [CrossRef]
[18]. Mathur S, Dubey T, Kulshrestha M, Agarwal H, Mathur G, Mathur A, Clinical profile and mortality among novel influenza A (H1N1) infected patients: 2009-2010 Jodhpur, Rajasthan pandemicJ Assoc Physicians India 2013 61(9):627-32. [Google Scholar]
[19]. Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, Pandemic H1N1 Influenza in Pregnancy Working Group. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United StatesJAMA 2010 303:1517-25.10.1001/jama.2010.47920407061 [Google Scholar] [CrossRef] [PubMed]
[20]. Patel M, Dennis A, Flutter C, Khan Z, Pandemic (H1N1) 2009 influenzaBr J Anaesth 2010 104(2):128-42.10.1093/bja/aep37510.1093/bja/aep375 [Google Scholar] [CrossRef] [PubMed]
[21]. ANZIC Influenza Investigators and Australasian Maternity Outcomes Surveillance System. Critical illness due to 2009 A/H1N1 influenza in pregnant and postpartum women: population based cohort study. BMJ. 2010;340:c127. Available from: https://www.ncbi.nlm.nih.gov/pubmed/2029969410.1136/bmj.c127920299694 [Google Scholar] [CrossRef] [PubMed]
[22]. Koul PA, Bali NK, Mir H, Jabeen F, Ahmad A, Influenza Illness in Pregnant Indian Women: A Cross-Sectional StudyInfect Dis Obstet Gynecol 2016 :124847010.1155/2016/124847026903762 [Google Scholar] [CrossRef] [PubMed]
[23]. Jiménez MF, Beitune PE, Salcedo MP, Ameln AV, Mastalir FP, Bravn LD, Outcomes for pregnant women infected with the influenza A (H1N1) virus during the 2009 pandemic in Porto Alegre, BrazilInt J Gynaecol Obstet 2010 111:217-19.10.1016/j.ijgo.2010.06.02420801449 [Google Scholar] [CrossRef] [PubMed]
[24]. Pramanick A, Rathore S, Peter JV, Moorthy M, Lionel J, Pandemic (H1N1) 2009 virus infection during pregnancy in South IndiaIntl J Gynaecol Obstet 2011 113:32-35.10.1016/j.ijgo.2010.10.02521315351 [Google Scholar] [CrossRef] [PubMed]
[25]. Koul PA, Bali NK, Ali S, Jabeen F, Ahmad A, Bhat MA, Poor uptake of influenza vaccination in pregnancy in northern IndiaInt J Gynaecol Obstet 2014 127(3):234-37.10.1016/j.ijgo.2014.05.02125085688 [Google Scholar] [CrossRef] [PubMed]
[26]. Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, Effectiveness of maternal influenza immunization in mothers and infantsN Engl J Med 2008 359(15):1555-64.10.1056/NEJMoa070863018799552 [Google Scholar] [CrossRef] [PubMed]