Introduction
Head and neck squamous cell carcinoma can be considered as solid tumours with heterogeneous content and usually have its origin in the epithelial tissue of the oral cavity, pharynx and larynx. A peculiar type of cancer cells has been found to be present in HNSCC and other tumours, similar in behaviour to tumour progenitor cells but having characteristic features consistent with CSCs [1,2]. The CSCs are defined as the cells in the tumour growth having a tumour initiating potential. There are three important characteristics of a normal stem cell:
Capacity to self-renew;
Ability to strictly control stem cell numbers;
Ability to divide and differentiate for generation of all functional properties of that tissue [3].
As compared to normal stem cells, the CSCs are known to have no control on the cell numbers. A very small number of CSCs are responsible for the growth of the tumour cells. The identification of the cell type capable of sustaining the neoplastic growth is one of the fundamental problems in cancer. It is evident that most of the cancers are clones and each cancer cell represents the progeny of one cell, but the cell type (CSCs) possessing the tumour-initiating cell function and the methods to recognise them is still unclear [4].
Recently, the CSCs were also shown to be present in solid tumours such as breast cancer and brain tumours [5,6]. The CSCs not only have self-renewal capability but also can generate a wide spectrum of progeny like normal stem cells [7]. Therefore, newer therapeutic approaches are likely to be developed through the identification of CSCs in HNSCC that regulate the growth, metastasis, and treatment resistance of tumour.
CSCs Origin
Two important factors are considered to recognise the origin of the CSCs [Table/Fig-1]:
Schematic diagram depicting the origin of cancer stem cell (CSC). Normal stem cells produce normal progenitor cells which undergoes genetic mutation to be transformed into cancer cells.
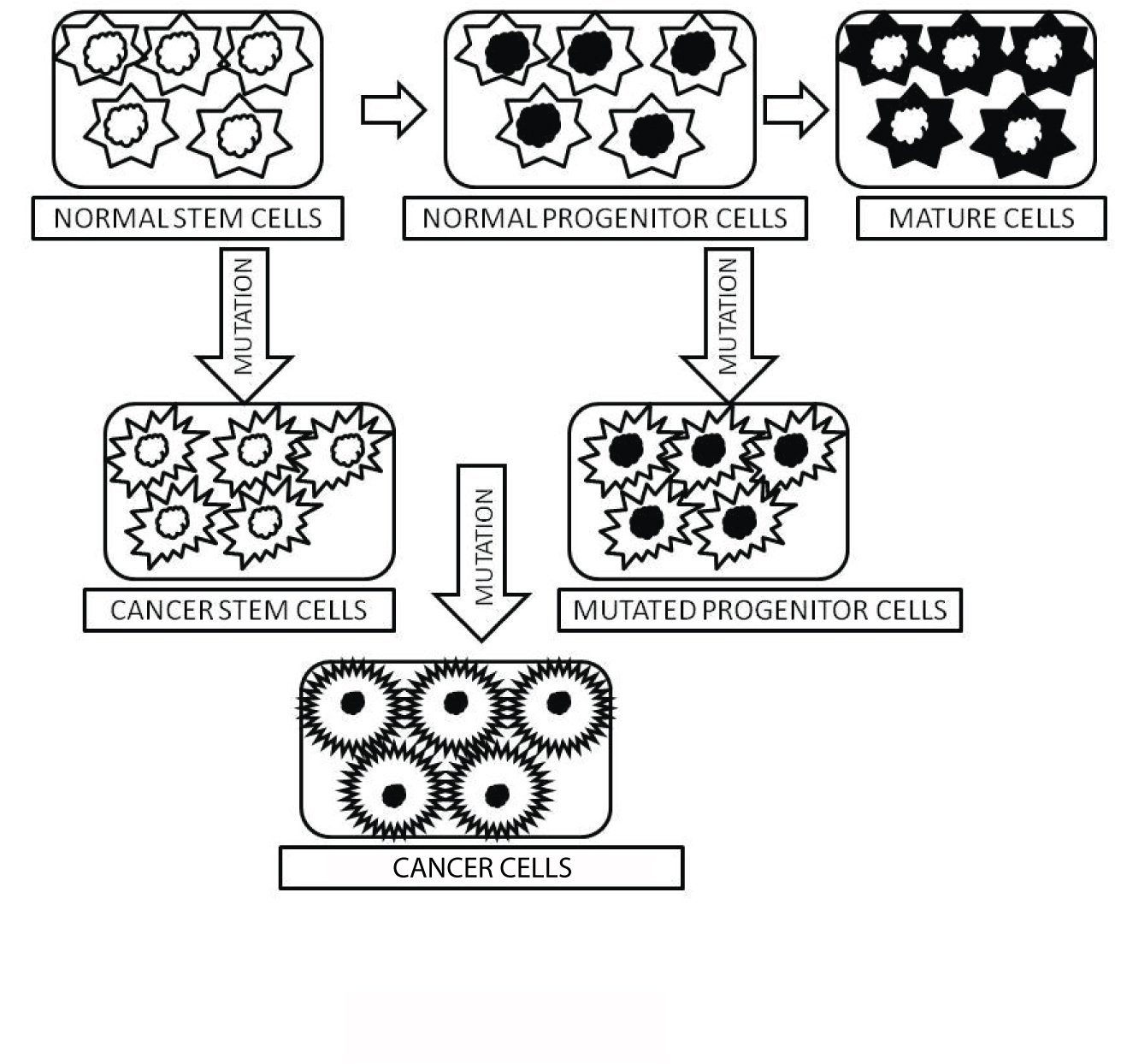
Cells are cancerous depending upon a number of mutations [8];
Absence of genetic constraints on both self-renewal and proliferation capabilities of CSCs [9];
Thus, CSCs can be obtained from either the self-renewing normal stem cells or from the progenitor cells with the ability of self-renewal due to mutations [10].
Outcomes for Cancer Treatment
There are three possibilities of cancer stem cells playing a role in tumourigenesis [Table/Fig-2]:
Pictorial representation showing the outcomes of cancer treatment. Primary tumour cells undergoing chemotherapy can eventually lead to the development of chemo-resistant cancer stem cells leading to recurrence of metastasis.
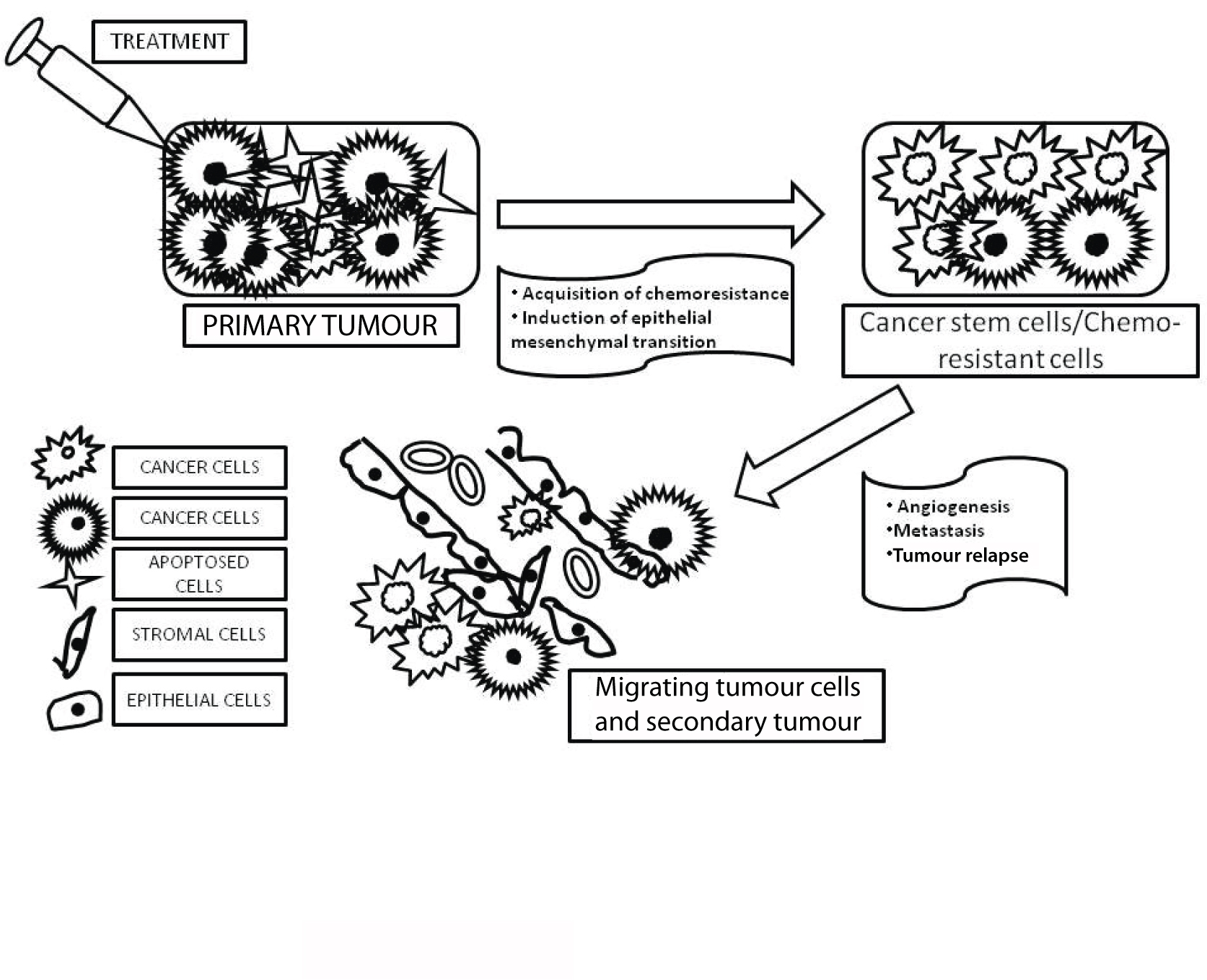
Development of the primary tumour due to mutation of normal stem cells or progenitor cells into CSCs.
Chemotherapy destroys most of the primary tumour cells but not CSCs which may become refractory; hence, may lead to recurrence of tumour.
CSCs may migrate from the primary tumour to distal sites leading to metastasis [11].
The CSCs are comparatively quiescent than other cancer cells. The absence of activated hyperproliferation signals such as tyrosine kinase makes the CSCs resistant to the toxic anticancer drugs that usually target the rapidly dividing cells.
CSCs Therapy
CSCs therapy includes cancer therapy by targeting the population of CSCs utilising several methods [Table/Fig-3]. Chemotherapy and radiotherapy are conventional therapy that are toxic to the healthy tissues and are not always able to kill CSCs, leading to multiple malignancies [12]. Moreover, by the process of Epithelial Mesenchymal Transition (EMT) in embryogenesis, CSCs can also promote metastatic characteristics of tumours [13-15]. Hence, CSCs therapy has become an important focus of cancer research to eliminate malignancies completely. There are various approaches that can target CSCs, most importantly the underlying cause that maintains it and can be utilised for new therapeutic strategies for head and neck cancer treatment. These approaches can be summarised as follows:
Diagrammatic representation of the current cancer therapies for head and neck squamous cell carcinoma targeting the population of cancer stem cells (CSC).
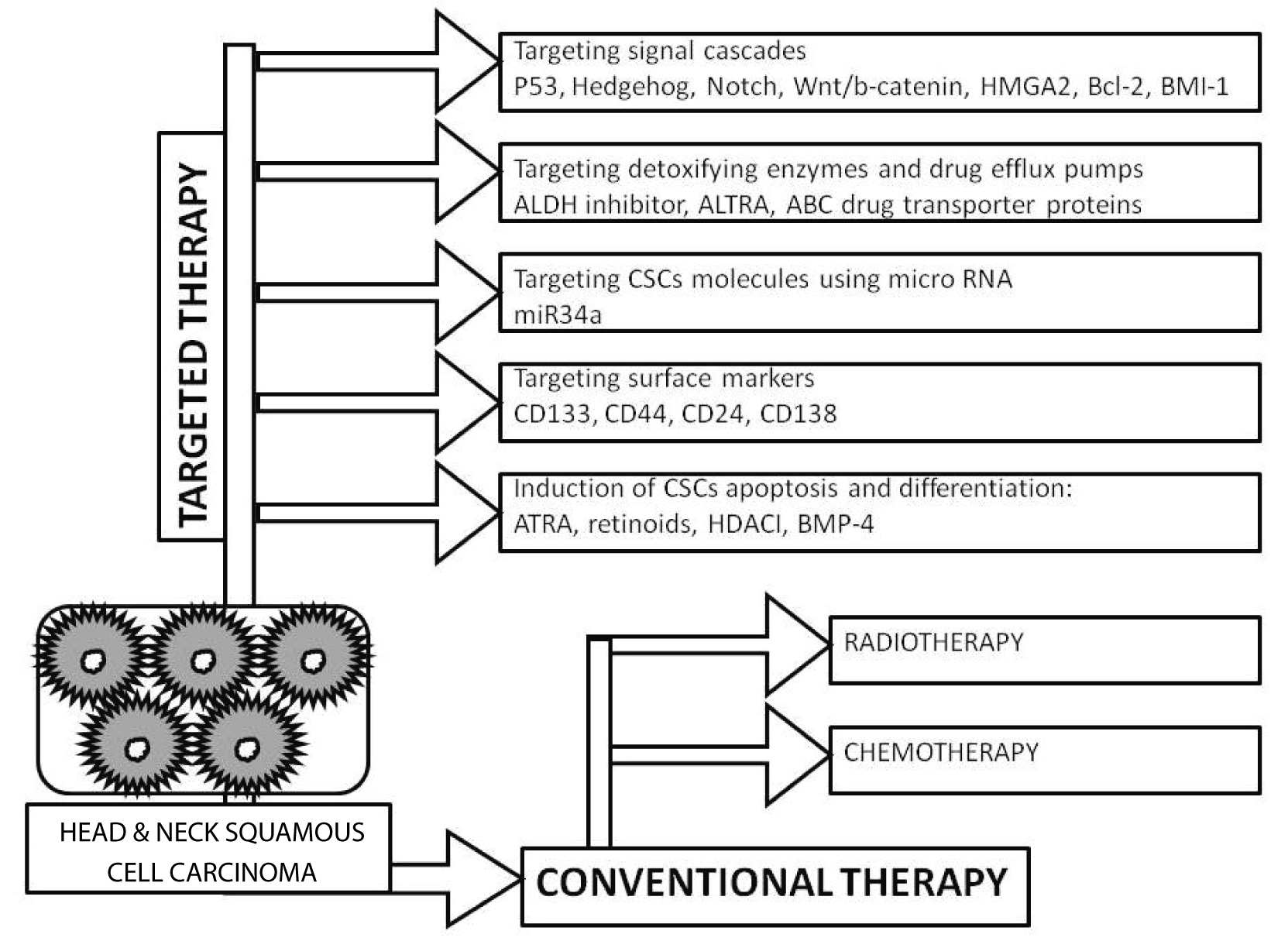
1. Targeting signalling pathways: The normal stem cells share common signalling pathways with CSCs like Hedgehog (Hh), Notch, Wnt/b-catenin, or High Mobility Group AT-hook 2 (HMGA2), B-cell lymphoma 2 (Bcl 2), B lymphoma Mo-MLV insertion region 1 homolog (BMI-1), and others that undergo aberrant activation or dysregulation to give rise to CSCs [16-18]. Amongst these, BMI-1 is considered as one of the important stem cell-related genes involved in the regulation of carcinogenesis in head and neck cancers. It was reported that the chemosensitivity of CSCs in HNSCC improved after knockdown of BMI-1 and CD44. Furthermore, the sensitivity of HNSCC cells increased to cisplatin on knockdown of CD44; thus, accounting for CSCs in response to chemotherapy [19]. This suggests that those anticancer drugs having the formulations of various inhibitors of such signalling pathways can be an answer to target the CSCs. However, the drawback of using such inhibitors is their adverse effect on the normal stem cells. Therefore, a combination of other CSCs-targeting therapies can also be used in anticancer drug formulations in order to improve their specificity.
2. Targeting detoxifying enzymes and drug efflux pumps: Certain CSCs are rich in drug detoxifying enzymes like Aldehyde Dehydrogenases (ALDH)-1. The importance of ALDH1 is its specificity as CSCs marker for the identification of highly tumourigenic cells present in HNSCC [20]. It has been shown that ALDH1+ CD44+ cells in HNSCC cells promote tumour propagation by resistance to radiotherapy and maintaining CSCs like properties [21]. Studies have also shown that the use of specific ALDH inhibitor Diethylamino-Benzaldehyde (DEAB) or All-Trans Retinoic Acid (ATRA) caused inhibition of ALDH1 activity in breast CSCs, resulting in reduced aggressiveness. Thus, the sensitivity of breast CSCs was increased towards chemotherapeutic drugs [22]. It was demonstrated earlier that the survival fraction of CSCs was decreased by UCN-01 (a checkpoint kinase inhibitor) in combination with ATRA with irradiation, thus suggesting for a powerful radio-sensitising strategy in HNSCC [23]. Moreover, the ATP Binding Cassette (ABC) drug transporter proteins common for both normal and CSCs are efflux pumps and protect the cells from xenotoxins. These pumps offer multiple drug resistance to the CSCs. It also has been reported that in lung cancer cells the use of pharmacological molecules in vitro and in vivo to target the ABC drug transporter proteins has led to increased sensitivity of CSCs to chemotherapy and radiotherapy [24].
3. Targeting CSCs molecules using micro-RNA: CSC markers can directly target certain micro-RNAs for their down-regulation. For example, miR-34a is one such micro-RNA which is a key negative regulator of CD44+ prostate cancer cells. This suggests the use of miR-34a as novel therapeutic agent against prostate CSCs [25].
4. Induction of CSCs apoptosis and differentiation: The resistance to chemotherapy and radiotherapy can be produced by anti-apoptotic proteins in CSCs such as Interleukin-4 (IL-4) produced by CD133+ colon carcinoma cells [26]. Experiments have shown that the sensitivity of CD133+ colon carcinoma cells to chemotherapeutic drugs (oxaliplatin and 5-fluorouracil) increased successfully by the use of IL-4 neutralising antibody and IL4 antagonists to target IL-4. Inducing the terminal differentiation of CSCs by agents like ATRA and retinoids, Histone Deacetylase Inhibitors (HDAI) and Bone Morphogenetic Protein 4 (BMP 4) can also be an approach to inhibit the self-renewal of CSCs instead of killing CSCs [27-29].
The origin of tumour may be accompanied by a deorganisation of the normal cellular structure of the tissue/organ and/or an increased proliferative potential known as anaplasia [30]. A number of morphological characteristics are usually considered to define anaplasia, including the following:
Abnormal nuclear morphology;
Loss of polarity;
Mitosis;
Pleomorphism;
Stromal alterations.
Characteristics of CSCs
1. Resistance to chemotherapy: There are various factors that contribute to drug resistance in cancers for e.g., glutathione and its enzyme structure like topoisomerase II, O6-methylguanine-DNA-methyltransferase, dihydrofolate reductase, metallothioneins, and ABC transporter proteins which are encoded by the multidrug resistant gene, the multidrug resistant protein, and the breast cancer resistant protein1 [31,32]. Therefore, it becomes crucial to investigate relationship between CSCs and these factors.
2. Resistance to irradiation: The ability of cancerous cells to survive and cause tumour recurrence suggests the property of radio-resistance of CSCs [33].
3. Invasion/metastatic activity: The ability of the malignant tumour cells to invade and disseminate into normal tissue help them to metastasise into other tissues. CSCs have high invasion activity as evident from the fact that even surgical operations cannot remove some of the infiltrating cancer cells and causes recurrence. This is also supported by the higher expression of CD44 and chemokines. Chemokine (C-X-C motif) receptor 4 (CXCR4) mediates cell migration in CD133+ cancer cells [34,35].
Preparation of CSCs
1. Cell surface markers: A useful way to separate CSCs from many types of tumours is by cell surface markers, such as CD133. Generally, CD133 molecule (a transmembrane pentaspan protein) is used as a marker of normal haematopoietic stem cells and organ-specific stem cells [36]. The expression of CSC markers in cancers differs in patterns, histological types and degrees of differentiation. The benefit of using such markers is the isolation of CSCs by Fluorescence-Activated Cell Sorting (FACS) and analysis of their biological characteristics for therapeutic purposes.
2. Sphere formation assay: The sphere forming methods are used by CSC researchers to concentrate CSCs in culture. However, it is better to use a monolayer culture method to characterise CSCs. The monolayer-cultured CSCs provide the advantage of being expanded as a homogeneous population [37].
3. Aldehyde dehydrogenase activity: ALDH is a detoxifying enzyme which blocks alkylating agents by oxidising intracellular aldehydes to carboxylic acids. It has been shown earlier that ALDH increases in Tumour Stem Cells (TSCs) [38,39]. Increasing evidence is available which reports that ALDH is expressed strongly by many types of CSCs and can be purified from tumours and cancer cell lines [40-42].
4. Side population: Studies have shown the presence of TSCs in both Side Population (SP) and non-SP and that stem cell marker are not expressed by SP cells [43,44]. It has been also demonstrated that only SP cells and not the non-SP cells possess the property of self-renewal in culture resistance to anti-cancer drugs including mitoxantrone, and formation of tumours when transplanted in vivo [45-50].
5. Niche for CSCs: The regulation of TSCs involves both intrinsic mechanism and extracellular signals which are derived from specialised microenvironment “niche”. This is proved by the fact that the ablation of such niche results in loss of TSCs. It appears that such niche is also required by CSCs for tumourigenesis. A premetastatic niche is formed by the bone-marrow derived progenitors before the arrival of cancer cells in the tumour specific premetastatic sites. This is evident from the fact that the ablation of such niche prevents tumour metastasis [51]. Experiments involving specific ablation of endothelial cells associated with tumour using inducible caspase-9 in Severe Combined Immunodeficiency (SCID) mouse model of human tumour angiogenesis showed that there is a decrease in fraction of head and neck CSCs [52]. Similarly, it has been also demonstrated that targeting the CSC niche produced more durable response in HNSCC; thus, developing a new concept of using both conventional chemotherapy and CSCs targeted therapy [53].
Signalling Pathways Involved in CSCs Maintenance
Genetic alterations cause maintenance of TSCs, amplification of precursors, or transformation of differentiated cells to CSCs.
1. p53 pathway: Studies have shown that loss of p53 function promotes accelerated cell proliferation and malignant transformation [54]. The p21 cyclin-dependent kinase (cdk) inhibitor which is considered as the effector of p53 regulates the progression of cells through the G1 cell-cycle phase. However, the use of p21 gene itself as an oncogenic target in human cancers has not been demonstrated.
2. Activation of receptor tyrosine kinase pathway: Signalling pathways of Receptor Tyrosine Kinases (RTKs) that play a role in maintenance of TSCs and amplification of precursors such as Platelet-Derived Growth Factor Receptor (PDGF), Epidermal Growth Factor receptor (EGF), fibroblast growth factor receptor, and insulin-like growth factor 1 receptor are frequently mutated in tumours [55].
3. Notch signalling pathway: Receptors play an important role in biological functions such as cell proliferation, differentiation, survival, and tumourigenesis [56]. Evidences are available which suggest that apart from maintaining the multi-potentiality of neural stem cells the notch activation plays a role in tumourigenesis.
4. Wnt signalling pathway: There are diverse developmental processes like cell proliferation and fate decisions that involves coordination of Wnt family of secreted proteins [57-59].
5. Hedgehog signalling pathway: The processes like proliferation, development, and tumourigenesis also involve Hh signalling. Studies have shown the involvement of ectopic activation of Hh signalling in tumour formation in the central nervous system (CNS), thus suggesting an important role of Hh signalling in brain tumourigenesis [60-62].
6. Stemness in tumour cells: The adult stem cells have a relatively long half-life and can suffer prolonged exposure to genotoxic stress during tumour development. This may cause accumulation of initial mutations leading to cancer [63].
7. Biomarkers in CSCs: There exists a difference in the expression of CSCs markers in cancers depending on its pattern, histological types and degrees of differentiation. Such markers are beneficial in the isolation of CSCs and analysis of their biological characteristics for effective therapeutic purposes.
8. CD133: Studies have shown the use of CD133 as a surface marker of CSCs in solid primary tumours like medullo-blastomas and glioblastomas, as well as cancers of epithelial tissues. CD133 molecule (a transmembrane pentaspan protein) is also considered as a universal marker of normal haematopoietic stem cells and organ-specific stem cells [64].
9. CD44: Several functions are attributed to the CD44 protein such as cell adhesion, motility, proliferation, drug resistance, and cell survival [65,66]. Experiments have also shown the role of CD44 in lymphocyte homing, wound healing, and cell migration, cancer cell growth and metastasis [67,68]. An immune-deficient mouse model was used in one of the first studies on CSCs in HNSCC. This study demonstrated that those CD44+ cancer cells that are responsible for formation of new tumours in vivo along with the ability of self-renewal and differentiation constitute only 10% of the cells in a HNSCC primary tumour. However, some of HNSCC expressing CD44s (standard form) and CD44 v6 (alternative splice variant), particularly in laryngeal cancers are associated with a poorer disease-free survival [69].
10. CD24: Recent studies have reported the role of CD24 in the developmental hedgehog pathway that is often active in CSCs [70].
11. CD138: Tumour cells that lack CD138 are capable of clonogenic growth and are relatively drug resistant. Such functional properties have also been attributed to CSCs in several human cancers [71].
12. Other CD molecules: Additionally, a list of other CD molecules in CSCs in various types of cancer is provided in the [Table/Fig-4] [72-79].
List of CD molecules related to various cancer types [72-79].
| Type of cancer | CD molecule |
|---|
| Prostate cancer | CD147+ [72] |
| Classic Hodgkin’s lymphoma | CD20+ [73] |
| Non-Hodgkin’s lymphoma | CD47+ [74] |
| Hepatocellular carcinoma | CD90+ [75] |
| Osteosarcoma | CD117+ [76] |
| Acute myeloid leukaemia | CD32+ or CD25+ or both [77], CD34+ CD38− [78] |
| Multiple myeloma | CD138− CD20+/ CD27+ [79] |
Conclusion
A better understanding of the resistance mechanisms of CSCs in HNSCC is required involving future studies for improving therapy and possibly preventing tumour spread or recurrence. The important roles of CSCs in progression of cancer can be evaluated by complementary in vitro approaches. Investigation of CSCs gives the idea of making novel targets for cancer that can be helpful in drug resistance and may combat the tumour cell metastasis [80]. When the patients are given therapy to cancer, great care must be taken as cancer stem cells are similar to normal stem cells. New and improved experimental approaches such as in vitro assay systems, will allow scientists to evaluate the relation between the different cell types within a tumour and their microenvironment.
[1]. Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinomaProc Natl Acad Sci USA 2007 104(3):973-78.10.1073/pnas.061011710417210912 [Google Scholar] [CrossRef] [PubMed]
[2]. Wicha MS, Liu S, Dontu G, Cancer stem cells: an old idea- -a paradigm shiftCancer Res 2006 66:1883-90.10.1158/0008-5472.CAN-05-315316488983 [Google Scholar] [CrossRef] [PubMed]
[3]. Bixby S, Kruger GM, Mosher JT, Joseph NM, Morrison SJ, Cell intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversityNeuron 2002 35(4):643-56.10.1016/S0896-6273(02)00825-5 [Google Scholar] [CrossRef]
[4]. Hope KJ, Jin L, Dick JE, Human acute myeloid leukemia stem cellsArch Med Res 2003 34(6):507-14.10.1016/j.arcmed.2003.08.00714734090 [Google Scholar] [CrossRef] [PubMed]
[5]. Dick JE, Breast cancer stem cells revealedProc Natl Acad Sci USA 2003 100:3547-49.10.1073/pnas.083096710012657737 [Google Scholar] [CrossRef] [PubMed]
[6]. Galderisi U, Cipollaro M, Giordano A, Stem cells and brain cancerCell Death Differ 2006 13:05-11.10.1038/sj.cdd.440175716123777 [Google Scholar] [CrossRef] [PubMed]
[7]. Al-Hajj M, Wicha MS, Ito-Hernandez A, Morrison SJ, Clarke MF, Prospective identification of tumourigenic breast cancer cellsProc Natl Acad Sci USA 2003 100(7):3983-88.10.1073/pnas.053029110012629218 [Google Scholar] [CrossRef] [PubMed]
[8]. Kopelovich L, Herbert BS, Heritable one-hit events defining cancer prevention?Cell Cycle 2013 12:2553-57.10.4161/cc.2569023907126 [Google Scholar] [CrossRef] [PubMed]
[9]. Morrison SJ, Qian D, Jerabek L, Thiel BA, Park IK, Ford PS, A genetic determinant that specifically regulates the frequency of haematopoietic stem cellsJ Immunol 2002 168:635-42.10.4049/jimmunol.168.2.63511777956 [Google Scholar] [CrossRef] [PubMed]
[10]. Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF, Therapeutic implications of cancer stem cellsCurr Opin Genet Dev 2004 14(1):43-47.10.1016/j.gde.2003.11.00715108804 [Google Scholar] [CrossRef] [PubMed]
[11]. Jordan CT, Guzman ML, Noble M, Cancer stem cellsN Engl J Med 2006 355(12):1253-61.10.1056/NEJMra06180816990388 [Google Scholar] [CrossRef] [PubMed]
[12]. Winquist RJ, Boucher DM, Wood M, Furey BF, Targeting cancer stem cells for more effective therapies: Taking out cancer’s locomotive engineBiochem Pharmacol 2009 78:326-34.10.1016/j.bcp.2009.03.02019539800 [Google Scholar] [CrossRef] [PubMed]
[13]. Chen C, Wei Y, Hummel M, Hoffmann TK, Gross M, Kaufmann AM, Evidence for epithelial-mesenchymal transition in cancer stem cells of head and neck squamous cell carcinomaPLoS One 2011 6(1):e1646610.1371/journal.pone.001646621304586 [Google Scholar] [CrossRef] [PubMed]
[14]. Davis SJ, Divi V, Owen JH, Bradford CR, Carey TE, Papagerakis S, Metastatic potential of cancer stem cells in head and neck squamous cell carcinomaArch Otolaryngol Head Neck Surg 2010 136:1260-66.10.1001/archoto.2010.21921173377 [Google Scholar] [CrossRef] [PubMed]
[15]. Sun S, Wang Z, Head neck squamous cell carcinoma c-Met+ cells display cancer stem cell properties and are responsible for cisplatin-resistance and metastasisInt J Cancer 2011 129(10):2337-48.10.1002/ijc.2592721225626 [Google Scholar] [CrossRef] [PubMed]
[16]. Maugeri-Saccà M, Zeuner A, De Maria R, Therapeutic targeting of cancer stem cellsFront Oncol 2011 1:1010.3389/fonc.2011.0001022655230 [Google Scholar] [CrossRef] [PubMed]
[17]. Muller JM, Chevrier L, Cochaud S, Meunier AC, Chadeneau C, Hedgehog, Notch and Wnt developmental pathways as targets for anti-cancer drugsDrug Discov Today: Disease Mechanism 2007 4:285-91.10.1016/j.ddmec.2008.05.009 [Google Scholar] [CrossRef]
[18]. Merchant AA, Matsui W, Targeting Hedgehog-a cancer stem cell pathwayClin Cancer Res 2010 16(12):3130-40.10.1158/1078-0432.CCR-09-284620530699 [Google Scholar] [CrossRef] [PubMed]
[19]. Chen YC, Chang CJ, Hsu HS, Chen YW, Tai LK, Tseng LM, Inhibition of tumourigenicity and enhancement of radio-chemosensitivity in head and neck squamous cell cancer-derived ALDH1-positive cells by knockdown of Bmi-1Oral Oncol 2010 46(3):158-65.10.1016/j.oraloncology.2009.11.00720036608 [Google Scholar] [CrossRef] [PubMed]
[20]. Chen YC, Chen YW, Hsu HS, Tseng LM, Huang PI, Lu KH, Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancerBiochem Biophy Res Commun 2009 385(3):307-13.10.1016/j.bbrc.2009.05.04819450560 [Google Scholar] [CrossRef] [PubMed]
[21]. Visus C, Ito D, Amoscato A, Maciejewska-Franczak M, Abdelsalem A, Dhir R, Identification of human aldehyde dehydrogenase 1 family member A1 as a novel CD8+ T-cell-defined tumour antigen in squamous cell carcinoma of the head and neckCancer Res 2007 67(21):10538-45.10.1158/0008-5472.CAN-07-134617974998 [Google Scholar] [CrossRef] [PubMed]
[22]. Croker AK, Allan AL, Inhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDHhiCD44+ human breast cancer cellsBreast Cancer Res Treat 2012 133(1):75-87.10.1007/s10549-011-1692-y21818590 [Google Scholar] [CrossRef] [PubMed]
[23]. Bertrand G, Maalouf M, Boivin A, Battiston-Montagne P, Beuve M, Levy A, Targeting head and neck cancer stem cells to overcome resistance to photon and carbon ion radiationStem Cell Rev 2014 10(1):114-26.10.1007/s12015-013-9467-y23955575 [Google Scholar] [CrossRef] [PubMed]
[24]. Xia X, Yang J, Li F, Li Y, Zhou X, Dai Y, Image-based chemical screening identifies drug efflux inhibitors in lung cancer cellsCancer Res 2010 70(19):7723-33.10.1158/0008-5472.CAN-09-436020841476 [Google Scholar] [CrossRef] [PubMed]
[25]. Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44Nat Med 2011 17(2):211-15.10.1038/nm.228421240262 [Google Scholar] [CrossRef] [PubMed]
[26]. Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Colon cancer stem cells dictate tumour growth and resist cell death by production of interleukin-4Cell Stem Cell 2007 1(4):389-402.10.1016/j.stem.2007.08.00118371377 [Google Scholar] [CrossRef] [PubMed]
[27]. Massard C, Deutsch E, Soria JC, Tumour stem cell-targeted treatment: elimination or differentiationAnn Oncol 2006 17(11):1620-24.10.1093/annonc/mdl07416600978 [Google Scholar] [CrossRef] [PubMed]
[28]. Butler LM, Zhou X, Xu WS, Scher HI, Rifkind RA, Marks PA, The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxinProc Natl Acad Sci USA 2002 99:11700-05.10.1073/pnas.18237229912189205 [Google Scholar] [CrossRef] [PubMed]
[29]. Lombardo Y, Scopelliti A, Cammareri P, Todaro M, Iovino F, Gulotta G, Bone morphogenetic protein 4 induces differentiation of colorectal cancer stem cells and increases their response to chemotherapy in miceGastroenterology 2011 140(1):297-309.10.1053/j.gastro.2010.10.00520951698 [Google Scholar] [CrossRef] [PubMed]
[30]. Stricker TP, Kumar V, NeoplasiaRobbins and Cotran Pathologic Basis of Disease 2010 8th edPhiladelphiaSaunders/Elsevier:259-330.10.1016/B978-1-4377-0792-2.50012-221168953 [Google Scholar] [CrossRef] [PubMed]
[31]. Gottesman MM, Fojo T, Bates SE, Multidrug resistance in cancer: role of ATP-dependent transportersNat Rev Cancer 2002 2(1):48-58.10.1038/nrc70611902585 [Google Scholar] [CrossRef] [PubMed]
[32]. Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM, Multidrug resistance in cancerNat Rev Drug Discov 2006 5(3):219-34.10.1038/nrd198416518375 [Google Scholar] [CrossRef] [PubMed]
[33]. Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Glioma stem cells promote radio-resistance by preferential activation of the DNA damage responseNature 2006 444(7120):756-60.10.1038/nature0523617051156 [Google Scholar] [CrossRef] [PubMed]
[34]. Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Distinct populations of cancer stem cells determine tumour growth and metastatic activity in human pancreatic cancerCell Stem Cell 2007 1(3):313-23.10.1016/j.stem.2007.06.00218371365 [Google Scholar] [CrossRef] [PubMed]
[35]. Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastomaMol Cancer 2006 5:67-78.10.1186/1476-4598-5-6717140455 [Google Scholar] [CrossRef] [PubMed]
[36]. Karbanová J, Missol-Kolka E, Fonseca AV, Lorra C, Janich P, Hollerová H, The stem cell marker cd133 (prominin-1) is expressed in various human glandular epitheliaJ Histochem Cytochem 2008 56(11):977-93. [Google Scholar]
[37]. Pollard SM, Yoshikawa K, Clarke ID, Danovi D, Striker S, Russell R, Glioma stem cell lines expanded in adherent culture have tumour-specific phenotypes and are suitable for chemical and genetic screensCell Stem Cell 2009 4(6):568-80.10.1369/jhc.2008.95189718645205 [Google Scholar] [CrossRef] [PubMed]
[38]. Tomita H, Tanaka K, Tanaka T, Hara A, Aldehyde dehydrogenase 1A1 in stem cells and cancerOncotarget 2016 7(10):11018-32.10.18632/oncotarget.692026783961 [Google Scholar] [CrossRef] [PubMed]
[39]. Cai J, Cheng A, Luo Y, Lu C, Mattson MP, Rao MS, Membrane properties of rat embryonic multipotent neural stem cellsJ Neurochem 2004 88(1):212-26.10.1046/j.1471-4159.2003.02184.x14675165 [Google Scholar] [CrossRef] [PubMed]
[40]. Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcomeCell Stem Cell 2007 1(5):555-67.10.1016/j.stem.2007.08.01418371393 [Google Scholar] [CrossRef] [PubMed]
[41]. Korkaya H, Paulson A, Iovino F, Wicha MS, HER2 regulates the mammary stem/progenitor cell population driving tumourigenesis and invasionOncogene 2008 27(47):6120-30.10.1038/onc.2008.20718591932 [Google Scholar] [CrossRef] [PubMed]
[42]. Pearce DJ, Taussig D, Simpson C, Allen K, Rohatiner AZ, Lister TA, Characterization of cells with a high aldehyde dehydrogenase activity from cord blood and acute myeloid leukaemia samplesStem Cells 2005 23(6):752-60.10.1634/stemcells.2004-029215917471 [Google Scholar] [CrossRef] [PubMed]
[43]. Mitsutake N, Iwao A, Nagai K, Namba H, Ohtsuru A, Saenko V, Characterization of side population in thyroid cancer cell lines: cancer stem-like cells are enriched partly but not exclusivelyEndocrinology 2007 148(4):1797-803.10.1210/en.2006-155317234707 [Google Scholar] [CrossRef] [PubMed]
[44]. Morita Y, Ema H, Yamazaki S, Nakauchi H, Non-side-population haematopoietic stem cells in mouse bone marrowBlood 2006 108(8):2850-56.10.1182/blood-2006-03-01020716804114 [Google Scholar] [CrossRef] [PubMed]
[45]. Haraguchi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, Barnard GF, Characterization of a side population of cancer cells from human gastrointestinal systemStem Cell 2006 24(3):506-13.10.1634/stemcells.2005-028216239320 [Google Scholar] [CrossRef] [PubMed]
[46]. Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, A distinct “side population” of cells with high drug efflux capacity in human tumour cellsProc Natl Acad Sci USA 2004 101(39):14228-33.10.1073/pnas.040006710115381773 [Google Scholar] [CrossRef] [PubMed]
[47]. Kondo T, Setoguchi T, Taga T, Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell lineProc Natl Acad Sci USA 2004 101:781-86.10.1073/pnas.030761810014711994 [Google Scholar] [CrossRef] [PubMed]
[48]. Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG, Side population is enriched in tumourigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumourigenicCancer Res 2005 65(14):6207-19.10.1158/0008-5472.CAN-05-059216024622 [Google Scholar] [CrossRef] [PubMed]
[49]. Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Isolation and in vitro propagation of tumourigenic breast cancer cells with stem/progenitor cell propertiesCancer Res 2005 65(13):5506-11.10.1158/0008-5472.CAN-05-062615994920 [Google Scholar] [CrossRef] [PubMed]
[50]. Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsivenessProc Natl Acad Sci USA 2006 103(30):11154-59.10.1073/pnas.060367210316849428 [Google Scholar] [CrossRef] [PubMed]
[51]. Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic nicheNature 2005 438(7069):820-27.10.1038/nature0418616341007 [Google Scholar] [CrossRef] [PubMed]
[52]. Krishnamurthy S, Dong Z, Vodopyanov D, Imai A, Helman JI, Prince ME, Endothelial cell-initiated signalling promotes the survival and self-renewal of cancer stem cellsCancer Res 2010 70(10):9969-78.10.1158/0008-5472.CAN-10-171221098716 [Google Scholar] [CrossRef] [PubMed]
[53]. Krishnamurthy S, Nör JE, Head and neck cancer stem cellsJ Dent Res 2012 91:334-40.10.1177/002203451142339321933937 [Google Scholar] [CrossRef] [PubMed]
[54]. Toledo F, Wahl GM, Regulating the p53 pathway: in vitro hypotheses, in vivo veritasNat Rev Cancer 2006 6(12):909-23.10.1038/nrc201217128209 [Google Scholar] [CrossRef] [PubMed]
[55]. Schubbert S, Shannon K, Bollag G, Hyperactive Ras in developmental disorders and cancerNat Rev Cancer 2007 7(4):295-308.10.1038/nrc210917384584 [Google Scholar] [CrossRef] [PubMed]
[56]. Radtke F, Raj K, The role of Notch in tumourigenesis: oncogene or tumour suppressor?Nat Rev Cancer 2003 3(10):756-67.10.1038/nrc118614570040 [Google Scholar] [CrossRef] [PubMed]
[57]. Logan CY, Nusse R, The Wnt signalling pathway in development and diseaseAnnu Rev Cell Dev Biol 2004 20:781-810.10.1146/annurev.cellbio.20.010403.11312615473860 [Google Scholar] [CrossRef] [PubMed]
[58]. Moon RT, Kohn AD, De Ferrari GV, Kaykas A, WNT and beta-catenin signalling: diseases and therapiesNat Rev Genet 2004 5(9):691-701.10.1038/nrg142715372092 [Google Scholar] [CrossRef] [PubMed]
[59]. Reya T, Clevers H, Wnt signalling in stem cells and cancerNature 2005 434(7035):843-50.10.1038/nature0331915829953 [Google Scholar] [CrossRef] [PubMed]
[60]. Pasca di Magliano M, Hebrok M, Hedgehog signalling in cancer formation and maintenanceNat Rev Cancer 2003 3(12):903-911.10.1038/nrc122914737121 [Google Scholar] [CrossRef] [PubMed]
[61]. Ruiz i Altaba A, Palma V, Dahmane N, Hedgehog-Gli signalling and the growth of the brainNat Rev Neurosci 2002 3(1):24-33.10.1038/nrn70411823802 [Google Scholar] [CrossRef] [PubMed]
[62]. Ruiz i Altaba A, Sanchez P, Dahmane N, Gli and hedgehog in cancer: tumours, embryos and stem cellsNat Rev Cancer 2002 2(2):361-72.10.1038/nrc79612044012 [Google Scholar] [CrossRef] [PubMed]
[63]. Lobo NA, Shimono Y, Qian D, Clarke MF, The biology of cancer stem cellsAnnu Rev Cell Dev Biol 2007 23:675-99.10.1146/annurev.cellbio.22.010305.10415417645413 [Google Scholar] [CrossRef] [PubMed]
[64]. Kusumbe AP, Mali AM, Bapat SA, CD133-expressing stem cells associated with ovarian metastases establish an endothelial hierarchy and contribute to tumour vasculatureStem Cells 2009 27(3):498-508.10.1634/stemcells.2008-086819253934 [Google Scholar] [CrossRef] [PubMed]
[65]. Marhaba R, Zoller M, CD44 in cancer progression: adhesion, migration and growth regulationJ Mol Histol 2004 35(3):211-31.10.1023/B:HIJO.0000032354.94213.6915339042 [Google Scholar] [CrossRef] [PubMed]
[66]. Afify A, Purnell P, Nguyen L, Role of CD44s and CD44v6 on human breast cancer cell adhesion, migration, and invasionExp Mol Pathol 2009 86(2):95-100.10.1016/j.yexmp.2008.12.00319167378 [Google Scholar] [CrossRef] [PubMed]
[67]. Ponta H, Sherman L, Herrlich PA, CD44: from adhesion molecules to signalling regulatorsNat Rev 2003 4(1):33-45.10.1038/nrm100412511867 [Google Scholar] [CrossRef] [PubMed]
[68]. Ishimoto T, Oshima H, Oshima M, CD44 (+) slow-cycling tumour cell expansion is triggered by cooperative actions of Wnt and prostaglandin E(2) in gastric tumourigenesisCancer Sci 2009 101(3):673-78.10.1111/j.1349-7006.2009.01430.x20028388 [Google Scholar] [CrossRef] [PubMed]
[69]. Staibano S, Merolla F, Testa D, Iovine R, Mascolo M, Guarino V, OPN/CD44v6 overexpression in laryngeal dysplasia and correlation with clinical outcomeBr J Cancer 2007 97:1545-51.10.1038/sj.bjc.660407017987038 [Google Scholar] [CrossRef] [PubMed]
[70]. Keysar S, Jimeno A, More than markers: biological significance of cancer stem cell defining-moleculesMol Cancer Ther 2010 9(9):2450-57.10.1158/1535-7163.MCT-10-053020716638 [Google Scholar] [CrossRef] [PubMed]
[71]. Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, Characterization of clonogenic multiple myeloma cellsBlood 2004 103(6):2332-36.10.1182/blood-2003-09-306414630803 [Google Scholar] [CrossRef] [PubMed]
[72]. Hao JL, Cozzi PJ, Khatri A, Power CA, Li Y, CD147/EMMPRIN and CD44 are potential therapeutic targets for metastatic prostate cancerCurrent Cancer Drug Targets 2010 10(3):287-93.10.2174/15680091079119019320370680 [Google Scholar] [CrossRef] [PubMed]
[73]. Oki Y, Younes A, Does rituximab have a place in treating classic hodgkin lymphoma?Curr Haematol Malig Rep 2010 5(3):135-39.10.1007/s11899-010-0052-z20490723 [Google Scholar] [CrossRef] [PubMed]
[74]. Chao MP, Alizadeh AA, Tang C, Mylebust JH, Varghese B, Gill S, Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphomaCell 2010 142(5):699-713.10.1016/j.cell.2010.07.04420813259 [Google Scholar] [CrossRef] [PubMed]
[75]. Lingala S, Cui YY, Chen X, Ruebner BH, Qian XF, Zern MA, Immuno histochemical staining of cancer stem cell markers in hepatocellular carcinomaExp Mol Pathol 2010 89(1):27-35.10.1016/j.yexmp.2010.05.00520511115 [Google Scholar] [CrossRef] [PubMed]
[76]. Adhikari AS, Agarwal N, Wood BM, Porretta C, Ruiz B, Pochampally RR, CD117 and Stro-1 identify osteosarcoma tumour-initiating cells associated with metastasis and drug resistanceCancer Res 2010 70(11):4602-12.10.1158/0008-5472.CAN-09-346320460510 [Google Scholar] [CrossRef] [PubMed]
[77]. Saito Y, Kitamura H, Hijikata A, Tomizawa-Murasawa M, Tanaka S, Takegi S, Identification of therapeutic targets for quiescent, chemotherapy- resistant human leukaemia stem cellsSci Transl Med 2010 2:17-19.10.1126/scitranslmed.300034920371479 [Google Scholar] [CrossRef] [PubMed]
[78]. Natasha YF, Markus HF, ABCB5 gene amplification in human leukaemia cellsLeuk Res 2009 33:1303-05.10.1016/j.leukres.2009.04.03519477512 [Google Scholar] [CrossRef] [PubMed]
[79]. Matsui W, Wang Q, Barber JP, Brennam S, Smith BD, Borrello I, Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistanceCancer Res 2008 68(1):190-97.10.1158/0008-5472.CAN-07-309618172311 [Google Scholar] [CrossRef] [PubMed]
[80]. Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CHM, Jones D L, Cancer stem cells-Perspectives on current status and future directions: AACR Workshop on Cancer Stem CellsCancer Research 2006 66(19):9339-44.10.1158/0008-5472.CAN-06-312616990346 [Google Scholar] [CrossRef] [PubMed]