Introduction
Epidemiology
Diabetes is becoming a disease of global concern because of its high escalating numbers. The number of diabetics is expected to increase from 382 million in 2013 to 592 million by 2035 [1]. Such a great rise in the number of patients represent a heavy disease burden at the individual and population level as well as for the total health care system.
The leading cause of morbidity and mortality for patients with type 2 diabetes is CVD. According to American Heart Association, at least 68% of the people with diabetes aged 65 or older die of heart disease and 16% die of stroke [2]. Furthermore, adults with diabetes are two to four times more likely to have CVD than adults without diabetes. Thus, it is considered as coronary artery disease equivalent [2].
The overall cardiometabolic risk is driven by a complex interplay between several non-modifiable (age, gender, genetics), modifiable (hypertension, hyperlipidemia, hyperglycemia) factors and the components of the metabolic syndrome commonly associated with type 2 diabetes [3]. Amongst all these factors, an entity called atherogenic dyslipidemia comprising of qualitative and quantitative changes in the lipoproteins like LDL, HDL and other Triglyceride Rich Lipoproteins (TRLs) play an important role [4]. This review aims to summarize recent advances in pathophysiology and management guidelines of diabetic dyslipidemia.
Pathophysiology
Different mechanisms are responsible for the development of dyslipidemia in patients with diabetes type 1 and type 2.
In type 1 diabetes, the levels of fasting plasma lipids and HDL is often normal or more favourable than age and gender matched normal patients [5]. This may in part relate to decreases in hepatic cholesterol synthesis in patients with type 1 diabetes. Despite this higher levels of LDL cholesterol and triglycerides still relate to CVD [6].
In type 2 diabetes, the typical pattern is that of the dyslipidemia of the metabolic syndrome with hypertriglyceridemia and reductions in HDL cholesterol. Other lipoprotein alterations include increases in LDL particle number, small dense LDL, and apolipoprotein (apoB) [7].
Insulin resistance/deficiency in type 2 diabetics in association with other factors like adipocytokines, hyperglycemia lead to qualitative, quantitative and kinetic changes in normal lipid metabolism [Table/Fig-1] including :
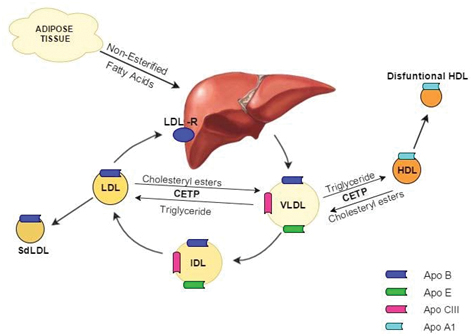
(1) Increased VLDL.
(2) Increased LDL.
(3) Decreased HDL [7].
1. Increased very low density lipoprotein:- It has been demonstrated that insulin plays a role in almost all the steps of Very Low Density Lipoprotein (VLDL) production and secretion.
A) Overproduction:- Recently it has been reported that overproduction of large VLDL1 particles resulting from increased production of both VLDL1-triglyceride and VLDL1 apoB is a key factor determining the concentration of serum triglycerides in subjects with type 2 diabetes [8].
Disturbance in triglyceride synthesis:- Triglyceride (TG) are derived from many processes like synthesis from (a) Free Fatty Acid (FFA) re-esterification, (b) De Novo Lipogenesis (DNL) and (c) Uptake of remnant particles [9].
The FFA availability is increased in insulin resistance states due to unabated lipolysis in adipose tissues as a result of the reduced inhibition of hormone sensitive lipase [6].
Accelerated DNL promoted by insulin resistance and hyperglycemia are being increasingly recognized as important contributors [10].
Increased apo B100:- Insulin resistant states in diabetics presumably activate endoplasmic reticulum based Microsomal Transfer Protein (MTP). The later then leads to assembling of TG with apoB100 preventing the degradation of apoB 100 [11].
(B) Increased secretion:- Increased secretion of VLDL probably results from increased expression of apo-CIII mediated through unopposed activity of Forkhead Box Protein 01 (FoxO1) in the liver in insulin resistant states [12].
(C) Decreased catabolism:- Reduced LPL activity stems from insulin deficiency and/or insulin resistance (activator of LPL) states present in diabetics. This leads to decreased catabolism of VLDL [13].
In addition to this quantitative change in VLDL, there is qualitative change seen as well in the form of increased synthesis of more atherogenic form of VLDL i.e. large VLDL1 particle size [14].
2. Increased small dense LDL:- In patients of type 2 diabetes, increased VLDL1 results in formation of small dense low density lipoproteins by a well explained mechanism:
Cholesteryl Ester Transfer Protein (CETP) mediated triglyceride movement from VLDL1 to LDL.
Lipoprotein mediated lipolysis of the triglyceride rich LDL into small dense LDL [15].
3. Reduced HDL:- Diabetics have increased catabolism of the small dense HDL (formed from VLDL1 via CETP) particles resulting in decreased concentration of HDL [16].
4. Postprandial Hyperlipidemia:- Postprandial hyperlipidemia is highly prevalent in diabetic patients [17]. This is mediated by several mechanisms:-
(A) Decreased LPL (lipoprotein lipase) activity:- This leads to increase in the residence time of triglycerides in circulation [18].
(B) Decreased clearance of TRL remnants:- Hepatic clearance of Triglyceride Rich Lipoprotein (TRL) remnants, is mediated by the Heparan Sulfate Proteoglycan (HSPG) syndecan-1 which is rapidly metabolised in diabetics due to increased induction of heparan Sulfate 6-O-Endo-Sulfatase 2 (SULF2) [19].
Newer insights into the pathophysiology
Role of Adiponectin:- Recently there has been interest in role of reduced levels of adipokines such as adiponectin as seen in insulin resistance states in the pathogenesis of dyslipidemia in diabetics. They have been thought to increase FFA availability for VLDL production [20].
Association with NAFLD:- Hepatic lipid homeostasis is regulated by the balance between the import and export of lipids, and an imbalance between these processes leads to increased VLDL secretion or lipid accumulation in hepatocytes as seen in Non-Alcoholic Fatty Liver Disease (NAFLD), a common finding in subjects with type 2 diabetes [21].
Causative role of remnant particles:- Remnant particles formed as a result of hydrolysis of triglyceride rich lipoproteins are rich in cholesteryl ester and thus cannot cross endothelium efficiently. Raised levels of these remnant particles as seen in diabetics may increase cardiovascular risk [22].
Low HDL; an innocent by stander:- Contrary to the common perception that cardiovascular risk results from low HDL, recent studies have suggested that HDL might just to be a marker rather than the proposed causual mechanism [23].
Management of Diabetic Dyslipidemia
Lipid Goals:- Various guidelines have proposed different goals for LDL and HDL in diabetics [Table/Fig-2].
Showing recommendations on LDL-c goals.
| National Cholesterol EducationProgram and Adult Treatment Panel III | American Association of ClinicalEndocrinologists |
|---|
DM and ASCVD LDL-C <70 mg/dL Non-HDL-C <100 mg/dL DM only LDL-C <100mg/dL Non-HDL-C <130mg/dL
| Extreme Risk LDL-C <55mg/dL Very High Risk LDL-C <70mg/dL Apo-B <80 mg/dL High Risk LDL-C <100mg/dL Apo-B <90mg/dL Moderate Risk LDL-C <100mg/dL Apo-B <90mg/dL Low Risk LDL-C <130mg/dL
|
DM: Diabetes Mellitus ASCVD: Atherosclerotic Cardiovascular Disease LDL: Low Density Lipoproteins HDL: High Density Lipoproteins
1. National Cholesterol Education Program and Adult Treatment Panel III:- These guidelines have put forth target LDL and HDL levels based on a patient’s risk of having a cardiovascular event in forthcoming 10 years [24].
2. American Association of Clinical Endocrinologists (AACE):- According to the new 2017 guidelines, individuals with type 2 diabetes (T2DM) should be considered as low risk (having no risk factors), medium risk (having <2 risk factors with 10 years risk <10%), high risk (having >2 risk factors with 10 years risk 10-20%, T2DM or Chronic Kidney Disease (CKD) with no risk factors), very high risk (acute coronary syndrome, carotid or peripheral vascular disease, 10 years risk >20%; diabetes or CKD with one or more risk factors; heterozygous familial hypercholesterolemia (HeFH) or extreme risk (with unstable angina, established CVD in patients with other comorbidities like T2DM, CKD stages 3/4, HeFH) and each category has different LDL cholesterol goals and are depicted in [Table/Fig-2] [25].
Treatment
(A) Non-pharmacological therapies:
1. Diet:- Dietary changes consist of reduced caloric intake that should include at least five servings per day of fruits and vegetables, at least six servings per day of grains with one-third as whole grains, fish and lean meat. Patients should limit intake of saturated fats, trans fats and cholesterol [26]. The diet should also include 2 gm/days of plant and 10–25 gm/days of soluble fiber [26].
2. Exercise:- Moderate intensity exercise lasting for 30 minutes, four to six times per week is the recommended exercise program by American Association of Clinical Endocrinologists (AACE) [26].
(B) Pharmacological
I) Cholesterol lowering Agents:-
1. Statins:- NCEP-ATP III, AACE, ADA and ACC/AHA guidelines all recommend statins as first-line therapy for dyslipidemia in diabetics [24,26-28]. High-intensity and moderate-intensity statin treatment is emphasized [Table/Fig-3].
Showing guidelines for statin therapy by different associations.
| Name of theAssociations | High Intensity StatinTherapy | Moderate intensityStatin Therapy |
|---|
| American DiabetesAssociation Standardsof Medical Care InDiabetes | DM and ASCVD any age DM, age 40-75 and 1 risk factor DM, age <40 or >75 and risk factor
| DM, age <40 or >75 and 1 risk factor DM, age >40 and no risk factors
|
| American College ofCardiology/American Heart Association BloodCholesterol Guidelines for ASCVD Prevention | DM and LDL >190 mg/dL DM, age 40-75 year, LDL 70-189 mg/dL and 10 year ASCVD risk >7.5%
| DM, age 40-75, LDL70-189 mg/dL 10 year ASCVD risk <7.5%
|
High-intensity statin therapy includes atorvastatin 80 mg once daily (40 mg may be used if 80 mg is not tolerated) and rosuvastatin 20–40 mg once daily [29].
Moderate-intensity statin therapy includes atorvastatin 10–20 mg once daily, fluvastatin 40 mg twice daily, lovastatin 40 mg once daily, pravastatin 40–80 mg once daily, rosuvastatin 5–10 mg once daily, simvastatin 20–40 mg once daily and pitavastatin 2–4 mg once daily [29].
Both ACC/AHA assess 10 year and lifetime risk of having a cardiovascular event based on age, sex, race, total cholesterol, HDL-c, blood presuure, diabetes status and smoking status [27].
2. Fibrates: They are peroxisome proliferator-activated receptor (PPAR)-α agonists, so they reduce triglycerides and modestly increase HDL cholesterol and lead a small decrease in cardiovascular mortality [30].
3. Ezetimibe:- This is a selective cholesterol absorption inhibitor, is an effective lipid-lowering agent when used as monotherapy and is useful in patients who are unable to tolerate statin therapy. Ezetimibe can also be used in combination with statin therapy for greater lipid-lowering efficacy [31].
4. Purified omega-3 fatty acids:- They lower triglycerides but they have little effect on HDL or LDL cholesterol. A recent trial of omega-3 fatty acids in patients with metabolic syndrome or type 2 diabetes disappointingly found no effect on CHD risk [32].
II) Antidiabetic Agents:- In addition to their glucose-lowering properties, antidiabetic agents that directly improve insulin resistance may have effects on lipid levels, especially TG levels [Table/Fig-4].
Showing the effects of glucose lowering drugs on lipid profile.
| S.No | Antidiabetic Agents | Effects on Lipids |
|---|
| 1. | Metformin [33] | LDL ↓HDL ↔ ←Total Cholesterol ↓↔Triglycerides ↓↔ |
| 2. | Gliclazide [33] | Total Cholesterol ↓Triglycerides ↓ |
| 3. | Glimepiride [33] | HDL ↔ ← |
| 4. | Pioglitazone [33] | HDL ←Total Cholesterol ←Triglycerides ↓ |
| 5. | Sitagliptin [34] | HDL ↔ ← |
| 6. | Vildagliptin [35] | HDL ↔ ← |
| 7. | Linagliptin [36] | No effect |
| 8. | Teneligliptin [37] | LDL ↓HDL ←Total Cholesterol ↓Triglycerides ↓ |
| 9. | Canagliflozin [38] | LDL ←HDL ←Total Cholesterol ←Triglycerides ← |
| 10. | Empagliflozin [34] | LDL ↔ ←HDL ↔ ←Total Cholesterol ↔ ← |
| 11. | Exenatide [39] | LDL ↔ ←HDL ↔ ←Total Cholesterol ↓↔Triglycerides ↓ |
| 12. | Liraglutide [40] | Triglycerides ↓ |
| 13. | Dulaglutide [41] | LDL ↓Total Cholesterol ↓Triglycerides ↓ |
↓: decrease, ←: increase ↔: no effect
III) Antihypertensive Agents:- Some antihypertensive agents are known to be lipid neutral (angiotensin-converting enzyme inhibitors, calcium-channel blockers, and angiotensin II receptor blockers) or lipid friendly (α-blockers). In patients with type 2 diabetes and dyslipidemia, a lipid-neutral/friendly antihypertensive would be expected to be associated with greater clinical benefits than a lipid-hostile antihypertensive such as a β-blocker or thiazide diuretic [42].
IV) Bile Acid Sequestrant:- Colesevelam, the bile acid sequestrant has been used in practice to reduce LDL levels as well as improve blood glucose levels in Type 2 diabetes patients [43].
Future of Dyslipidemia Management:- Many new drugs have emerged for the treatment of dyslipidemia. However, their role in improving lipid profile in association of diabetes is yet to be studied.
I) PCSK9 inhibitors:- Recently, PCSK9 inhibitors like evolocumab and alirocumab have shown very good reductions in LDL levels in patients with high cardiovascular risk including type 2 diabetes. They should be considered in patients with clinical CVD who are unable to reach LDL-C/non- HDL-C goals with maximally tolerated statin therapy [44]. However, at present they are priced too high for use in routine clinical practice.
II) Saroglitazar:- It is a PPAR -α/γ agonist and has been shown to significantly reduce plasma triglyceride, total cholesterol, non-HDL cholesterol, and VLDL cholesterol, and HbA1c and fasting glucose levels [45].
III) Other drugs:- Some new LDL lowering drugs like thyroid mimetics, antisense oligonucleotides or Microsomal Transfer Protein Inhibitors (MTPI), triglyceride lowering drugs like peroxosimal proliferator activating receptors agonists, diacylglycerol acyl transferase-1 inhibitors, HDL lowering drugs mimetic peptides, modulators of inflammation (e.g. phospholipase inhibitors) and gene therapy in rare diseases (e.g. lipoprotein lipase deficiency) are in their initial stages.
Conclusion
Various hypotheses have been put forward to explain the typical diabetic dyslipidemia, summary of evidences point out that the overproduction of VLDL1 particles initiates a sequence of events that results in the atherogenic lipid triad consisting of elevated plasma concentrations of fasting and post-prandial TRLs, small dense LDL and low HDL cholesterol. However, the potential links between anti-inflammatory biomarkers like adiponectin and accumulation of hepatic fat remains is to be explored further. Though, various recommendations have been put forward, the therapeutic interventions for dyslipidemia are only complementary to the basic recommendations of adequate control of blood glucose, blood pressure and various other lifestyle modifications. Though, various treatment guidelines and all the available drugs for treatment of diabetic dyslipidemia have been discussed here, physicians are advised to individualize the treatment as per the characteristics and needs of specific patients using these guidelines.
[1]. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE, Global estimates of diabetes prevalence for 2013 and projections for 2035Diabetes Res Clin Pract 2014 103(2):137-49.10.1016/j.diabres.2013.11.00224630390 [Google Scholar] [CrossRef] [PubMed]
[2]. Cardiovascular Disease and Diabetes. American Heart Association. Available from Website: http://www.heart.org/HEARTORG/Conditions/Diabetes/WhyDiabetesMatters/Cardiovascular-Disease-Diabetes_UCM_313865_Article.jsp/#.VqfJL5orKUk Accessed on 04/01/2017 [Google Scholar]
[3]. Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23)BMJ 1998 316(7134):823-28.10.1136/bmj.316.7134.8239549452 [Google Scholar] [CrossRef] [PubMed]
[4]. Eliasson B, Cederholm J, Eeg-Olofsson K, Svensson AM, Zethelius B, Gudbjörnsdottir S, Clinical usefulness of different lipid measures for prediction of coronary heart disease in type 2 diabetes: a report from the Swedish National Diabetes RegisterDiabetes Care 2011 34(9):2095-100.10.2337/dc11-020921775750 [Google Scholar] [CrossRef] [PubMed]
[5]. Miettinen TA, Gylling H, Tuominen J, Simonen P, Koivisto V, Low synthesis and high absorption of cholesterol characterize type1 diabetesDiabetes Care 2004 27(1):53-58.10.2337/diacare.27.1.5314693966 [Google Scholar] [CrossRef] [PubMed]
[6]. Forrest KY, Becker DJ, Kuller LH, Wolfson SK, Orchard TJ, Are predictors of Coronary heart disease and lower-extremity arterial disease in type1 diabetes the same? A prospective studyAtherosclerosis 2000 148(1):159-69.10.1016/S0021-9150(99)00217-8 [Google Scholar] [CrossRef]
[7]. Mazzone T, Chait A, Plutzky J, Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studiesLancet 2008 371(9626):1800-09.10.1016/S0140-6736(08)60768-0 [Google Scholar] [CrossRef]
[8]. Adiels M, Borén J, Caslake MJ, Stewart P, Soro A, Westerbacka J, Overproduction of VLDL1 driven by hyperglycemia is a dominant feature of diabetic dyslipidemiaArterioscler Thromb Vasc Biol 2005 25(8):1697-703.10.1161/01.ATV.0000172689.53992.2515947244 [Google Scholar] [CrossRef] [PubMed]
[9]. Otero YF, Stafford JM, McGuinness OP, Pathway-selective insulin resistance and metabolic disease: the importance of nutrient fluxJ Biol Chem 2014 289(30):20462-469.10.1074/jbc.R114.57635524907277 [Google Scholar] [CrossRef] [PubMed]
[10]. Vedala A, Wang W, Neese RA, Christiansen MP, Hellerstein MK, Delayed secretory pathway contributions to VLDL-triglycerides from plasma NEFA, diet, and de novo lipogenesis in humansJ. Lipid Res 2006 47(11):2562-74.10.1194/jlr.M600200-JLR20016929033 [Google Scholar] [CrossRef] [PubMed]
[11]. Blasiole DA, Davis RA, Attie AD, The physiological and molecular regulation of lipoprotein assembly and secretionMol Bio Syst 2007 3(9):608-19.10.1039/b700706j17700861 [Google Scholar] [CrossRef] [PubMed]
[12]. Haas ME, Attie AD, Biddinger SB, The regulation of ApoB metabolism by insulinTrends Endocrinol Metab 2013 24(8):391-97.10.1016/j.tem.2013.04.00123721961 [Google Scholar] [CrossRef] [PubMed]
[13]. Taskinen MR, Nikkila EA, Kuusi T, Harmo K, Lipoprotein lipase activity and serum lipoproteins in untreated type 2 (insulinindependent) diabetes associated with obesityDiabetologia 1982 22(1):46-50.10.1007/BF002538697037508 [Google Scholar] [CrossRef] [PubMed]
[14]. Wang J, Stancakova A, Soininen P, Kangas AJ, Paananen J, Kuusisto J, Lipoproteinsubclass profiles in individuals with varying degrees of glucose tolerance: a population-based study of 9399 Finnish menJ Intern Med 2012 272(6):562-72.10.1111/j.1365-2796.2012.02562.x22650159 [Google Scholar] [CrossRef] [PubMed]
[15]. Packard CJ, Triacylglycerol-rich lipoproteins and the generation of small, dense low-density lipoproteinBiochem Soc Trans 2003 31(pt5):1066-69.10.1042/bst031106614505481 [Google Scholar] [CrossRef] [PubMed]
[16]. Frénais R, Ouguerram K, Maugeais C, Mahot P, Maugere P, Krempf M, High density lipoprotein apolipoprotein AI kinetics in NIDDM: a stable isotope studyDiabetologia 1997 40(5):578-83.10.1007/s0012500507189165227 [Google Scholar] [CrossRef] [PubMed]
[17]. Watts GF1, Barrett PH, Ji J, Serone AP, Chan DC, Croft KD, Differential regulation of lipoprotein kinetics by atorvastatin and fenofibrate in subjects with the metabolic syndromeDiabetes 2003 52(3):803-11.10.2337/diabetes.52.3.80312606523 [Google Scholar] [CrossRef] [PubMed]
[18]. Taskinen MR, Lipoprotein lipase in diabetesDiabetes Metab Rev 1987 3(2):551-70.10.1002/dmr.56100302083552532 [Google Scholar] [CrossRef] [PubMed]
[19]. Williams KJ, Chen K, Recent insights into factors affecting remnant lipoprotein uptakeCurr Opin Lipidol 2010 21(4):218-28.10.1097/MOL.0b013e328338cabc20463470 [Google Scholar] [CrossRef] [PubMed]
[20]. Vergès B, Abnormal hepatic apolipoprotein B metabolism in type 2 diabetesAtherosclerosis 2010 211(2):353-60.10.1016/j.atherosclerosis.2010.01.02820189175 [Google Scholar] [CrossRef] [PubMed]
[21]. Adiels M, Taskinen MR, Packard C, Caslake MJ, Soro-Paavonen A, Westerbacka J, Overproduction of large VLDL particles is driven by increased liver fat content in manDiabetologia 2006 49(4):755-65.10.1007/s00125-005-0125-z16463046 [Google Scholar] [CrossRef] [PubMed]
[22]. Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, Common variants associated with plasma triglycerides and risk for coronary artery diseaseNat. Genet 2013 45(11):1345-52. [Google Scholar]
[23]. Nordestgaard BG, Langsted A, Freiberg JJ, Nonfastinghyperlipidemia and cardiovascular diseaseCurr Drug Targets 2009 10(4):328-35.10.2174/13894500978784643419355857 [Google Scholar] [CrossRef] [PubMed] [CrossRef]
[24]. Grundy SM, Cleeman JI, Merz CN, Brewer HB, Clark LT, Hunninghake DB, Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III GuidelinesCirculation 2004 110(2):227-39.10.1161/01.CIR.0000133317.49796.0E15249516 [Google Scholar] [CrossRef] [PubMed]
[25]. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Available from https://www.aace.com/files/lipidguidelines.pdf. Accessed on 02/01/2017 [Google Scholar]
[26]. Jellinger PS, Smith DA, Mehta AE, Ganda O, Handelsman Y, Rodbard HW, American Association of Clinical Endocrinologists’ Guidelines for Management of Dyslipidemia and Prevention of AtherosclerosisEndocr Pract 2012 18(suppl 1):S1-78.10.4158/EP.18.S1.122522068 [Google Scholar] [CrossRef] [PubMed]
[27]. 2013 Prevention Guideline Tools: CV Risk Calculator. American Heart Association Available from: http://my.american heart.org/cvriskcalculator. Accessed 28/12/201610.2337/dc15-S00325537706 [Google Scholar] [CrossRef] [PubMed]
[28]. American Diabetes AssociationStandards of medical care in diabetes—2015Diabetes Care 2015 38(suppl 1):S1-99.10.2337/dc15-S00325537706 [Google Scholar] [CrossRef] [PubMed]
[29]. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guide-linesJ Am Coll Cardiol 2014 63(25 Pt B):2889-934. [Google Scholar]
[30]. Jun M, Foote C, Lv J, Neal B, Patel A, Nicholls SJ, Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysisLancet 2010 375(9729):1875-84.10.1016/S0140-6736(10)60656-3 [Google Scholar] [CrossRef]
[31]. Ballantyne CM, Houri J, Notarbartolo A, Melani L, Lipka LJ, Suresh R, Effect of ezetimibeco-administered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trialCirculation 2003 107(19):2409-15.10.1161/01.CIR.0000068312.21969.C812719279 [Google Scholar] [CrossRef] [PubMed]
[32]. Weintraub H, Update on marine omega-3 fatty acids: Management of dyslipidemia and current omega-3 treatment optionsAtherosclerosis 2013 230(2):381-89.10.1016/j.atherosclerosis.2013.07.04124075771 [Google Scholar] [CrossRef] [PubMed]
[33]. Buse JB, Tan MH, Prince MJ, Erickson PP, The effects of oral anti-hyperglycaemic medications on serum lipid profiles in patients with type 2 diabetesDiabetes Obes Metab 2004 6(2):133-56.10.1111/j.1462-8902.2004.00325.x14746579 [Google Scholar] [CrossRef] [PubMed]
[34]. Roden M, Weng J, Eilbracht J, Delafont B, Kim G, Woerle HJ, Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trialLancet Diabetes Endocrinol 2013 1(3):208-19.10.1016/S2213-8587(13)70084-6 [Google Scholar] [CrossRef]
[35]. Matikainen N, Manttari S, Schweizer A, Ulvestad A, Mills D, Dunning BE, Vildagliptin therapy reduces postprandial intestinal triglyceride-rich lipoprotein particles in patients with type 2 diabetesDiabetologia 2006 49(9):2049-57.10.1007/s00125-006-0340-216816950 [Google Scholar] [CrossRef] [PubMed]
[36]. Zinman B, Ahren B, Neubacher D, Patel S, Woerle HJ, Johansen OE, Efficacy and cardiovascular safety of linagliptin as an add-on to insulin in type 2 diabetes: a pooled comprehensive post hoc analysisCan J Diabetes 2016 40(1):50-57.10.1016/j.jcjd.2015.06.01026474870 [Google Scholar] [CrossRef] [PubMed]
[37]. Maladkar M, Sankar S, Kamat K, Teneligliptin: Heralding Change in Type 2 DiabetesJournal of Diabetes Mellitus 2016 6(2):113-31.10.4236/jdm.2016.62012 [Google Scholar] [CrossRef]
[38]. Forst T, Guthrie R, Goldenberg R, Yee J, Vijapurkar U, Meininger G, Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazoneDiabetes Obes Metab 2014 16(5):467-77.10.1111/dom.1227324528605 [Google Scholar] [CrossRef] [PubMed]
[39]. Schwartz EA, Koska J, Mullin MP, Syoufi I, Sachwenke DC, Reaven PD, Exenatide suppresses postprandial elevations in lipids and lipoproteins in individuals with impaired glucose tolerance and recent onset type 2 diabetes mellitusAtherosclerosis 2010 212(1):217-22.10.1016/j.atherosclerosis.2010.05.02820557887 [Google Scholar] [CrossRef] [PubMed]
[40]. Vilsboll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, Courrèges JP, Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetesDiabetes Care 2007 30(6):1608-10.10.2337/dc06-259317372153 [Google Scholar] [CrossRef] [PubMed]
[41]. Edwards KL, Minze MG, Dulaglutide: an evidence-based review of its potential in the treatment of type 2 diabetesCore Evid 2015 10:11-21.10.2147/CE.S5594425657615 [Google Scholar] [CrossRef] [PubMed]
[42]. Papadakis JA, Ganotakis ES, Jagroop IA, Mikhailidis DP, Winder AF, Effect of hypertension and its treatment on lipid, lipoprotein(a), fibrinogen, and bilirubin levels in patients referred for dyslipidemiaAm J Hypertens 1999 12(7):673-81.10.1016/S0895-7061(99)00049-7 [Google Scholar] [CrossRef]
[43]. Fonseca VA, Handelsman Y, Staels B, Colesevelam lowers glucose and lipid levels in type 2 diabetes: the clinical evidenceDiabetes Obes Metab 2010 12(5):384-92.10.1111/j.1463-1326.2009.01181.x20415686 [Google Scholar] [CrossRef] [PubMed]
[44]. McDonagh M, Peterson K, Holzhammer B, Fazio S, A Systematic Review of PCSK9 Inhibitors Alirocumab and EvolocumabJ Manag Care Spec Pharm 2016 22(6):641-53.10.18553/jmcp.2016.22.6.64127231792 [Google Scholar] [CrossRef] [PubMed]
[45]. Joshi SR, Saroglitazar for the treatment of dyslipidemia in diabetic patientsExpert Opin Pharmacother 2015 16(4):597-06.10.1517/14656566.2015.100989425674933 [Google Scholar] [CrossRef] [PubMed]