Surgery, although essential for tumour cancer treatment, suppresses immunity and therefore promotes metastasis. Surgical trauma elicits profound physiological changes that involve metabolic, inflammatory, and immune reactions and lead to widespread changes in organ functions [1,2]. The overall effect is commonly referred to as the stress response to surgery [3]. The surgical stress response occurs through the activation of the afferent neural and sympathetic nervous systems, and via potent inflammatory mediators, including cytokines [4,5]. The specific anaesthesia approach has shown that the choice of techniques and anaesthetic drugs severely influences the immune response and consequently causes cancer metastasis. Cytokines secreted by various cells initiate immunological and inflammatory reactions. Pro-inflammatory and anti-inflammatory cytokines are balanced in a healthy person; however, when excessive amounts of pro-inflammatory cytokines are secreted during trauma, sepsis, and neoplasms, this balance is lost, resulting in increased morbidity and mortality [6]. Thus, the suppression of harmful inflammatory responses related to surgery and anaesthesia is an important approach for improving surgical outcome.
Fentanyl is a synthetic agonist Mu receptor that is more potent than morphine (80-100 times). This results in rapid passage across the blood-brain barrier, therefore leading to quick onset after intravenous administration (2-3 minutes) and short duration of action (45-60 minutes) from rapid redistribution to inactive tissues [7].
Dexmedetomidine is a potent alpha-2 adrenoreceptor agonist, offers significant pharmacological properties such as sedation, anxiolysis and analgesia with the unique characteristic to cause no respiratory depression. In addition it possesses sympatholytic and antinociceptive effects that allow haemodynamic stability during surgical stimulation [8]. Several studies have demonstrated its safety, although bradycardia and hypotension are the most predictable and frequent side effects [9,10]. Dexmedetomidine has shown to consistently reduce opioids, propofol, and benzodiazepines requirements [8-11]. Dexmedetomidine may modulate the production of cytokines during the stress response [12,13]. The dexmedetomidine significantly lower the level of cytokines (IL-1b, TNF-β, and IL-10) intraoperative and postoperative period. Additionally, the IL-6 and IL-4 level were reduced by dexmedetomidine intraoperative and postoperative period as compared before surgery. The anti-inflammatory effect of dexmedetomidine may be useful in various clinical contexts [14].
The low concentration of C-reactive Protein (CRP) might have been caused by decrease in the levels of pro-inflammatory cytokines, induce the production of acute phase proteins, enhance cell-mediated immunological responses and increase the chemotaxis of inflammatory cells [15]. The anti-inflammatory effects of dexmedetomidine may be related to its central sympatholytic effects and relative stimulation of the cholinergic anti-inflammatory pathway [16,17]. Dexmedetomidine decreases Heart Rate (HR) and blood pressure due to its central sympatholytic effects. Recent evidence indicates that pain and immune factors, especially proinflammatory cytokines, mutually interact and influence each other and responsible for decrease the demand of rescue analgesia. However, the comparative effects of opioid sparing of anaesthesia on the postoperative cytokine response in oral cancer surgery in the Indian population, has as yet not been reported, to the best of our knowledge. Therefore, in the present study, we aim to compare the effects of fentanyl and dexmedetomidine with paracetamol administered by intravenous route on the postoperative cytokine response in oral cancer surgery.
Materials and Methods
Study Designs
This prospective comparative study was carried out after obtaining the approval from the institutional Ethical Committee. The patients of ASA grade I or II, age 18 to 70 years were included in this study. The study was carried out in the Surgical Department of K.G’s Medical University, Lucknow between September 2015 to August 2016. Informed consent was obtained from all the patients. Those patients with history of adverse reaction to any study medication, unstable cardiovascular disease, acute pulmonary diseases, history of heart block and hypertension and chronic pain syndrome were excluded from the study. Patients were randomly divided into two groups (n= 30 in each group) by computer generated random number table.
In Group Ist, patients received Paracetamol (PCM) 10 mg/kg body weight as premedication and fentanyl 2 μg/kg body weights at the time of induction and patients were induced with propofol 2 mg/kg body weight. Succinylcholine 2 mg/kg body weight iv was given and patients were intubated with appropriate size endotracheal tube. Maintenance of anaesthesia was done with oxygen, N2O, Isoflurane and non depolarising muscle relaxant vecuronium. Fentanyl 1 μg/kg was continued in perioperative period. Residual paralysis was reversed with neostigmine and glycopyrrolate.
In Group IInd, patients received PCM 10 mg/kg and dexmedetomidine 0.5 μg/kg in 100 mL normal saline in 10 minutes at the time of induction and patients were induced with propofol 2 mg/kg body weight. Succinylcholine 2 mg/kg body weight was administered and patients were intubated with appropriate size endotracheal tube. Dexmedetomidine 0.4 μg/kg was continued in perioperative period. Maintenance of anaesthesia was done with oxygen, N2O, isoflurane and non depolarising muscle relaxant vecuronium. Residual paralysis was reversed with neostigmine and glycopyrrolate.
Preoperative clinical assessment (haemodynamic, oxygen saturation, respiratory rate capnography, ECG and urine output) of the patients was done. An intravenous assessment was established with 18 gauze cannula. Patients were monitored routinely with Electrocardiography (ECG), non-invasive blood pressure and pulseoximetry every 5 minutes for 30 minute and then every 15 minute till the end of surgery and after two hour. All patients were premedicated with injection. glycopyrrolate 0.01 mg/kg iv and injection. Ondansetron 0.1 mg/kg iv. Bradycardia was defined as heart rate <50 beats/min for which Injection. Atropine 0.01 mg/kg was given. If mean arterial pressure was decreased ≥20% from baseline it was managed with fluid and ionotropic.
Sample Collection
Venous blood samples of 3 mL were collected separately using aseptic precautions preoperatively, postoperative and 24 hours after surgery. Blood sample was allowed to clot for 30 minutes and centrifuged. Serum was aliquoted and stored at -200C until testing.
Serum IL-6 level was determined by Ray bio human IL-6 ELISA kit which is an in vitro Enzyme Linked Immunosorbent Assay (ELISA) for the quantitative measurement of human IL-6 in serum. This assay employ an antibody specific for human IL-6 coated on a 96 well plates. Standard and samples are pipetted into the wells and IL-6 present in a sample is bound to the wells by immobilized antibody. The wells are washed and biotinylated anti human IL-6 antibody was added. After washing away unbound biotinylated antibody, Horseradish peroxidase (HRP)-conjugated streptavidin is pipetted to the wells. The wells are again washed; a Tetra Methyl Benzidine (TMB) substrate solution is added to the wells and colour developed in proportion to the amount of IL-6 bound. The stop solution changed the colour from blue to yellow and intensity of colour is measured at 450 nm. All laboratory tests were conducted by the same staff.
The CRP test was done in the Department Of Pathology by Sunred BIO CRP kit. They used a double-antibody sandwich ELISA to assay the level of rabbit CRP in samples. C-reactive protein was added to rabbit CRP monoclonal antibody pre-coated well, incubate and then biotin with Streptavidin-HRP labeled antibodies was added to form immune complex; then incubation was done and it washed again to remove the uncombined enzyme. Chromogen solution was added A, B, the color of the liquid changes into the blue, and due to acid, the color finally becomes yellow. The chroma of color and the concenthumanion of the rabbit substance CRP of sample were positively correlated.
Statistical Analysis
All statistical analyses were performed using SPSS version 15.0 windows software. Comparisons between groups at different time intervals were assessed by using student t-test. All the categorical data was compared by using chi-square test. Parametric data was compared using chi-square test. A p<0.05 was considered to be significant.
Results
The demographic profiles age, weight, duration of surgery, ASA grade and tumour stage were similar in both groups as shown in [Table/Fig-1].
Demographic profile of the patients.
| Group A(n=30) | Group B(n=30) | p-value |
|---|
| Age (Mean±SD) years | 57.73±8.54 | 58.57±8.48 | 0.706 |
| Sex |
| Female | 4 (13.33%) | 1 (3.33%) | 0.161 |
| Male | 26 (86.67%) | 29 (96.67%) |
| Weight (Mean±SD) kg | 59.23±5.93 | 59.17±5.06 | 0.963 |
| Duration of surgery (minutes) | 169.00±15.22 | 171.50±14.57 | 0.518 |
| Clinical-pathological status of oral cancer |
| I | 1 (3.3%) | 2 (6.7%) | 0.523 |
| II | 17 (56.7%) | 19 (63.3%) |
| III | 12 (40.0%) | 8 (26.7%) |
| IV | 0 (0.0%) | 1 (3.3%) |
| ASA Grade |
| I | 4 (13.33%) | 1 (3.33%) | 0.161 |
| II | 26 (86.67%) | 29 (96.67%) |
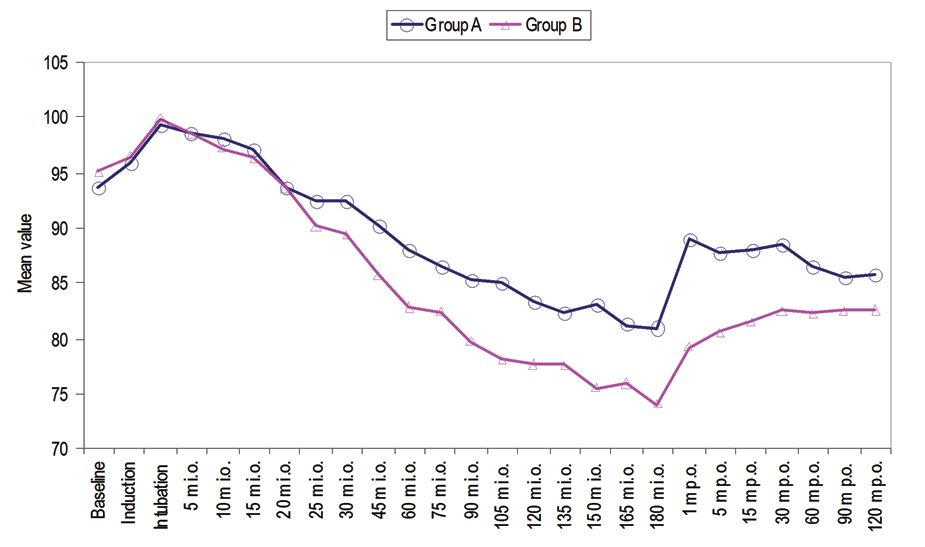
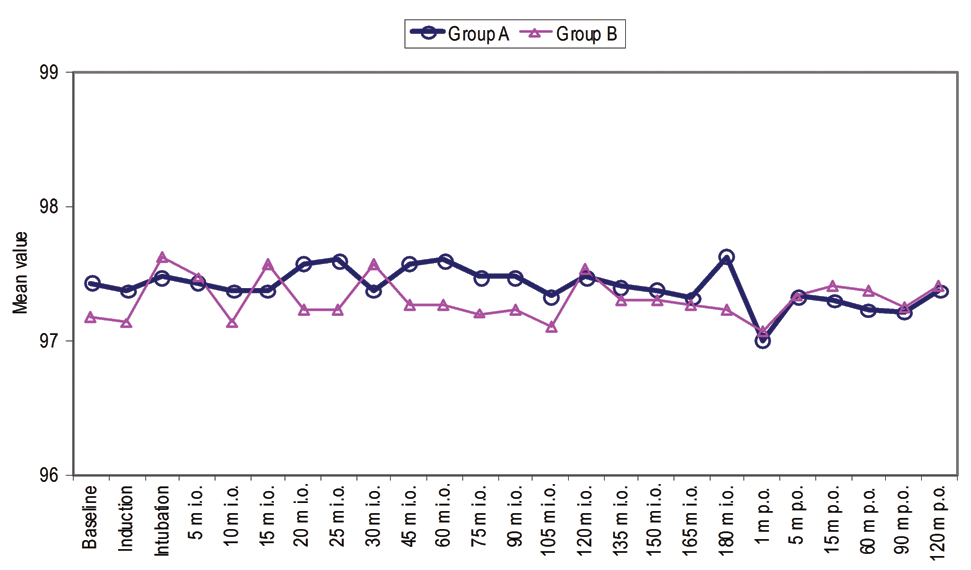
Mean Arterial Pressure (MAP) was observed at intubation which continuously declined thereafter and at 20-25 minute intraoperative MAP was found to be below the baseline. Thereafter, at all the time periods of observation MAP values were found to be below baseline. Post-surgery MAP values were noted one minute, five minute, 15 minute, 30 minute, 60 minute, 90 minute and 120 minute. At all these post-surgery time periods of observation, MAP values were found to be lower than that at baseline [Table/Fig-2]. The mean heart rate of group A and group B were not found to be statistically significant at induction (101.47±8.38 vs. 97.07±10.79 beats/min) and intubation (105.93±7.70 vs. 100.33±13.91 beats/min). At rest of the periods of observation except 120 minute postoperative mean heart rate of patients of group A was found to be statistically significantly higher (p<0.05) than those of group B [Table/Fig-3]. Oxygen saturation of all the patients during the observation period remained >95%. No statistically significant difference in oxygen saturation between the two groups was observed at any time period of observation (p>0.05) [Table/Fig-4].
Intraoperative and Postoperative MAP.
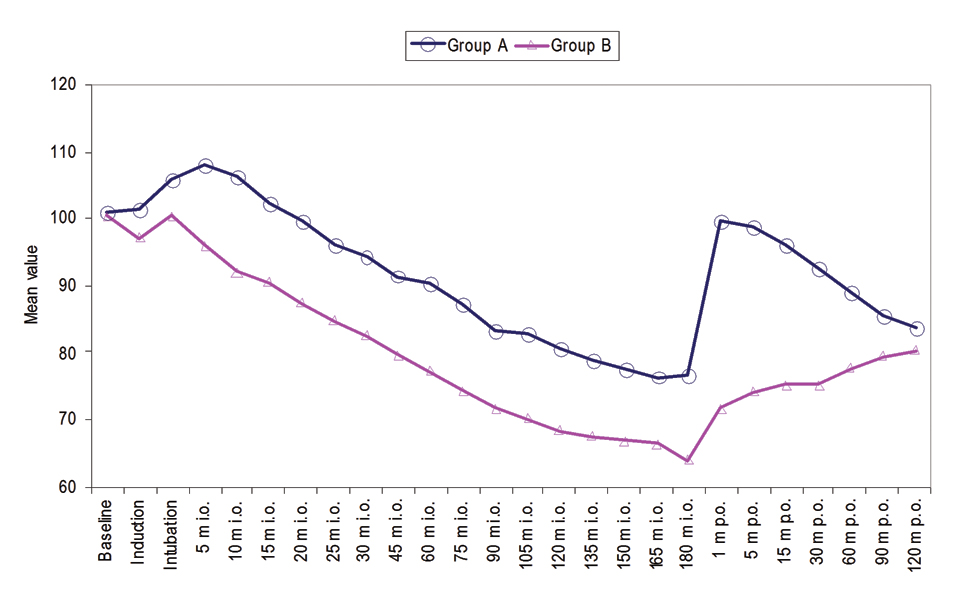
Intraoperative and postoperative oxygen saturation.
C-reactive protein level was not significantly different in between group A and group B preoperatively and postoperatively. Moreover, the CRP levels after 24 hours postoperative was also not significantly different in between group A (24.93±2.81 mg/dL) and group B (24.00±2.93 mg/dL) as shown in [Table/Fig-5]. A preoperative IL-6 level of group A was found to be higher than that of group B (24.13±3.59 vs. 24.00±2.42 pg/mL). Similarly, a postoperative IL-6 level of group A was found to be higher than that of group B (28.51±2.86 pg/mL vs. 27.97±2.27 pg/mL). At 24 hours postoperative, IL-6 levels of group A was found to be higher than that of group B (20.62±2.76 pg/mL vs. 20.30±1.73 pg/mL). At none of the time periods difference in mean IL-6 of group A and group B was found to be statistically significant (p>0.05) shown in [Table/Fig-5].
CRP and IL-6 levels of the patients group A and group B at baseline, immediate postoperative and 24-hour postoperative intervals.
| Biomarkers | Group A (n=30) Mean±SD | Group B (n=30) Mean±SD | p-value |
|---|
| CRP Level (mg/dL) |
| Preoperative | 19.86±2.53 | 18.96±1.95 | 0.128 |
| Post- operative | 23.50±2.62 | 24.22±2.22 | 0.259 |
| At 24-hr Postoperative | 24.93±2.81 | 24.00±2.93 | 0.216 |
| IL-6 Level (pg/mL) |
| Preoperative | 24.13±3.59 | 24.00±2.42 | 0.867 |
| Postoperative | 28.51±2.86 | 27.97±2.27 | 0.415 |
| At 24-hr Postoperative | 20.62±2.76 | 20.30±1.73 | 0.593 |
In group A, between preoperative and postoperative and between preoperative and 24-hours postoperative intervals, a mean change of CRP level 3.64±1.37 (18.33%) and 5.07±2.05 (25.53%) was observed, at both the time intervals, the change was significant statistically (p<0.001). Moreover, the mean CRP level change (1.43±2.27 mg/dL) was significantly different between postoperative and 24-hour postoperative intervals in group A [Table/Fig-6]. The mean change in CRP level 5.26±1.67 (27.74%) and 5.04±3.09 (26.58%) were significantly (p<0.001) different between preoperative and postoperative and between preoperative and 24-hour postoperative intervals in group B. Moreover, the mean change in CRP level (-0.22) was not significantly (p<0.001) different between postoperative and 24-hours postoperative intervals in group B [Table/Fig-6].
Changes in CRP and IL-6 levels in group A and group B between different periods.
| Group A (n=30) | Group B (n=30) |
|---|
| Mean Changes (%) | p-value | Mean Changes (%) | p-value |
|---|
| Changes in CRP Level at different time intervals |
| Preoperative to Postoperative | 3.64 (18.33%) | <0.001** | 5.26 (27.74%) | <0.001** |
| Preoperative to 24-hr post- operative | 5.07 (25.53%) | <0.001** | 5.04 (26.58%) | <0.001** |
| Postoperative to 24-hr postoperative | 1.43 (6.09%) | 0.01* | -0.22 (0.91%) | 0.702 |
| Changes in IL-6 Level at different time intervals |
| Preoperative to Postoperative | 4.38 (18.17%) | <0.001** | 3.97 (16.54%) | <0.001** |
| Preoperative to 24-hr postoperative | -7.89 (-27.68%) | <0.001** | -7.67 (-27.41%) | <0.001** |
| Postoperative to 24-hr postoperative | -3.51 (-14.55%) | <0.001** | -3.70 (-15.40%) | <0.001** |
*= significant (p<0.05), **= significant (p≤0.001)
In group A, an increment in IL-6 levels was observed during preoperative and postoperative period (4.38±1.56 pg/mL) and this change was found to be statistically significant (p<0.001) while a decrease in IL-6 levels after 24 hours of surgery and that at postoperative period was observed (7.89±3.63 pg/mL) change in IL-6 levels between immediate postoperative and 24 hours was also found to be statistically significant. At 24 hours postoperative, IL-6-levels were found to be lower than that at preoperative period (3.51±4.21 pg/mL) and this change was found to be statistically significant (p<0.001). In group B, an increment in IL-6 levels was observed during preoperative and postoperative period (3.97±0.92 pg/mL) and this change was found to be statistically significant (p<0.001) while a decrease in IL-6 levels after 24 hours of surgery and that at postoperative period was observed (7.67±2.25 pg/mL) change in IL-6 levels between immediate postoperative and 24 hours was also found to be statistically significant. At 24 hours postoperative, IL-6-levels were found to be lower than that at preoperative period (3.70±2.40 pg/mL) and this change was found to be statistically significant (p<0.001) as shown in [Table/Fig-6].
Immediate postoperative pain score (VAS) of group A (0.83±0.83) was found to be statistically significant higher (p=0.028) than that of group B (0.37±0.49). At eight hours postoperative pain score (VAS) of group A (5.37±0.72) was found to be statistically significant higher (p<0.001) than that of group B (4.33±0.48). At 24 hours postoperative difference in pain score (VAS) of group A (4.93±0.69) and group B (4.37±0.49) was found to be statistically significant [Table/Fig-7].
VAS Scores in between group A and group B.
| Group A (n=30) Mean±SD | Group B (n=30) Mean±SD | p-value |
|---|
| Immediate postoperative | 0.83±0.83 | 0.37±0.49 | 0.028 |
| 8-hr postoperative | 5.37±0.72 | 4.33±0.48 | <0.001** |
| 24-hr postoperative | 4.93±0.69 | 4.37±0.49 | 0.001** |
**= significant (p≤0.001)
Postoperative requirement of analgesia in majority of patients of group B was 0-1 times (76.7%) while postoperative requirement of analgesia in majority of patients of group A was two to three times. Difference in postoperative requirement of analgesia of above two groups was found to be statistically significant (p=0.001) as shown in [Table/Fig-8].
Evaluation of postoperative 24-hr analgesic requirement between group A and group B.
| No. of times Analgesia required | Group ANo.(%) | Group B (n=30) No.(%) | p-value |
|---|
| 0 | 0 (0.0%) | 9 (30.0%) | p<0.001** |
| 1 | 1 (3.3%) | 14 (46.7%) |
| 2 | 16 (53.3%) | 7 (23.3%) |
| 3 | 13 (43.3%) | 0 (0.0%) |
The complications such as hypotension, bradycardia, nausea and shivering were present in both the groups. Hypotension and bradycardia were higher in group B while nausea and shivering were higher in group A. Comparing the proportion of each complication between the three groups, χ2 test revealed similar (p>0.05) proportion of complications among the groups.
Discussion
In the present study, there was no significant difference in MAP at induction, intubation, 5, 10, 15, 20 and 25 minutes among the groups. However, there was significant difference in MAP among the groups at 30 minutes to 180 minutes intraoperative period and 90 minutes. postoperatively. However, a significant difference was found in Heart Rate (HR) among the groups from 5 min. intraoperative to 90 min. postoperatively, 30, 45 and 90 minutes. The HR was observed to be lowest in group B and highest in Group A. The heart rate in group A and group B were decreased over the periods as compared to baseline and decreased was significantly greater in group B. Uysal HY et al., and Bilgi KV et al., both were similarly found a reduced haemodynamic response to intubation with dexmedetomidine compared with fentanyl [18,19]. Our observations are supported by various studies which reported that the preoperative administration of dexmedetomidine resulted in progressive increase of sedation, blunted the haemodynamic responses during laryngoscopy, intubation, extubation and reduced opioid and anaesthetic requirements. Previous studies by various investigators also showed reduced stress response to this stimulus by administration dexmedetomidine in healthy normotensive patients [20-22].
Many previous studies, including that by Patel CR et al., found a significant decrease and less variability in the HRs of patients who received dexmedetomidine, and a low and stable systolic and diastolic Blood Pressures (BPs) as well [23]. However, Patel CR et al., had administered the maintenance dose of dexmedetomidine while the others administered a 1 μg/kg loading dose alone [23]. Lawrence and De Lange had also demonstrated lower perioperative serum catecholamine concentrations in the dexmedetomidine group [20].
In hypertensive patients, extubation is challenging today. During emergency, any sympathetic response from anaesthesia can increase morbidity [19]. In our study, by continuing a maintenance infusion of the drug throughout surgery, we achieved lower but comparable HRs at extubation in both the groups. Though, the hypertensive response of dexmedetomidine was significantly lower as compared to the baseline unlike that seen with fentanyl, indicating better control of blood pressure compared to fentanyl [19]. Previous study suggested that dexmedetomidine 0.5 μg/kg IV, administered before extubation, was more effective in attenuating airway reflex responses to tracheal extubation and maintaining haemodynamic stability without prolonging recovery compared with fentanyl 1 μg/kg IV in these patients undergoing rhinoplasty [24].
In this study, the fentanyl and dexmedetomidine groups were similar during the time to recovery from anaesthesia and extubation after stopping the infusion. Moreover, in dexmedetomidine group, the patients were recovered smoothly without any agitation and vital was more stable during extubation. Our results are supported by similar findings of previous study by Patel CR et al., who found that the dexmedetomidine attenuates various stress responses during surgery and maintains the haemodynamic stability when used as an adjuvant in general anaesthesia [23]. Also, the sedative action of dexmedetomidine delays recovery for the first few hours post extubation.
In our study the oxygen saturation (SpO2) was not significantly different in between fentanyl and dexmedetomidine group, intra and postoperatively. The findings of our study were supported by Sunil BV et al., and Shukla D et al., who reported that the arterial oxygen saturation, there was no significant difference between the fentanyl and dexmedetomidine groups through the measuring intervals [25,26].
In the present study, the VAS was significantly increased eight hours and 24 hours postoperatively as compared to immediately postoperatively. Moreover, the VAS was significantly lower in dexmedetomidine group as compared to fentanyl group during eight hours and 24 hours postoperatively. Our findings are supported by the studies of Feld JM et al., and Arain SR et al., who reported that the pain scores and morphine use were decreased in the dexmedetomidine group [27,28]. Dexmedetomidine provides intense analgesia during the postoperative period and reduces the total number of post-surgical patients requiring opioids with a corresponding reduction in opioid-associated side effects [29].
In the present study, we observed that the postoperative requirement of analgesia was found to be statistically significant in between groups. In dexmedetomidine group, the postoperative requirement of analgesia was significantly reduced as compared to fentanyl group. This shows that dexmedetomidine is better analgesic than fentanyl. Similarly, Feld JM et al., showed that the requirement of analgesia was lower in dexmedetomidine as compared to fentanyl, postoperatively [27]. The dexmedetomidine treated patients required significantly less morphine to achieve equivalent analgesia, they found that the use of dexmedetomidine for postoperative analgesia resulted in significantly less additional pain medication [28].
In the present study, we observed that the IL-6 level was not statistically different in between group A and group B at preoperative, postoperative and 24 hours postoperatively. IL-6 level was significantly lower in groups postoperatively at 24 hours as compared to preoperatively and immediate postoperatively. Moreover, the immediate postoperatively IL-6 level was significantly increased as compared to preoperatively in the groups. Helmy SA et al., reported that the pre-incisional, IL-6 did not show a significant change whereas it was significantly increased by the end of anaesthesia and surgery and decreased by 24-hour postoperatively [30].
In this study, we observed that the CRP level was not significantly different in between group A and group B at preoperative, postoperative and 24 hours postoperatively. The CRP level was significantly higher in groups, immediate postoperatively and 24 hours postoperatively as compared to preoperatively. Moreover, the immediate postoperatively and 24 hours postoperatively CRP level was not significantly different in the groups. Syeda T et al., demonstrated that the CRP value reaches a peak on the second postoperative day and declines after wards [31]. Preoperative serum CRP levels are associated with advanced tumour stage, bone invasion, lymph node extra-capsular spread, lymph node metastasis, and patients' survival. After craniotomy, CRP rapidly increased to reach a peak mean value on the second postoperative day. From days three to five after surgery, mean CRP values constantly and significantly declined (p<0.001) to arrive at a mean of 6.67±10.80 mg/l) on the fifth postoperative day [32].
In this study, there was no significant difference in adverse effect between the groups, but in dexmedetomidine group, three patients had hypotension and five patients had bradycardia as compared to fentanyl, in which one patient had bradycardia and two hypotension. Patient doesn’t require any medication. Bradycardia and hypotension corrected after stopping the drug for some time. In fentanyl group have three patients complain of nausea and two of shivering. While in dexmedetomidine group one patient have nausea and only one patient have shivering. Our findings are supported by other studies, in which they reported that there was no significant difference in adverse effect between dexmedetomidine and fentanyl [32-34].
Limitation
The sample size may have been too small, and multi-centered study with larger sample size would be required for better result.
Conclusion
In this study, we found that the dexmedetomidine is effective drug to be used as intravenous adjuvants for patients undergoing wild local excision and modified neck dissection surgery. It maintains haemodynamic stability and reduced the serum CRP level, postoperative pain and analgesic requirements as compared to fentanyl.
*= significant (p<0.05), **= significant (p≤0.001)
**= significant (p≤0.001)