A Case of Immunotactoid Glomerulopathy in a Patient with Multiple Myeloma
Irina G Rekhtina1, Larisa P Mendeleeva2, Patricia E Povivaitite3
1 Leader Research Scientist, Department of Nephrology, National Research Center for Hematology of the Ministry of Healthcare of The Russian Federation, Moscow.
2 Professor, Department of High-Dose Chemotherapy of Paraproteinemic Hemoblastoses, National Research Center for Hematology of the Ministry of Healthcare of the Russian Federation, Moscow.
3 Head, Department of Pathology, Regional Bureau of Pathology, Rostov of the Russian Federation.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Irina Rekhtina, Leader Research Scientist, Department of Nephrology, National Research Center for Hematology of the Ministry of Healthcare of the Russian Federation, Moscow. 125167 Novi Zikovski pr. 4, Moscow, Russia.
E-mail: rekhtina.i@blood.ru
Cases of Immunotactoid Glomerulopathy (ITG) in patients with Multiple Myeloma (MM) are extremely rare. Consequently, data on efficacy and therapy results are limited. We report on a rare case of long-term follow up patient with MM and ITG. After chemotherapy the patient achieved complete remission with complete recovery of renal function. A repeat renal biopsy revealed complete resorption of the deposits. After four years, ITG was diagnosed again in another kidney biopsy during a relapse of MM. There was excessive glomerular infiltration by lymphoid cells and obstruction of capillary lumen was identified. Bundles of microtubules, similar to the deposits in kidneys, were revealed in some lymphoid cells.
Glomerular infiltration, Glomerulonephritis, Microtubules
Case Report
A 56-year-old woman presented with macro-haematuria in March 2009. A physical examination revealed pale skin, lower leg oedema and arterial hypertension (BP 170/100 mmHg). Haemoglobin was 7.9 gm/dL, platelet count was 83,000/μL, creatinine level was 248 μmol/L, proteinuria was 4.7 gm of protein/24 h with no Bence Jones protein. Paraprotein Gκ (11.9 gm/L) was identified from serum protein electrophoresis/immunofixation. Cryoglobulinemia was absent. The bone marrow contained 15% plasma cells. Skeletal X-rays showed diffuse osteoporosis and there were no pathologies of the urinary system.
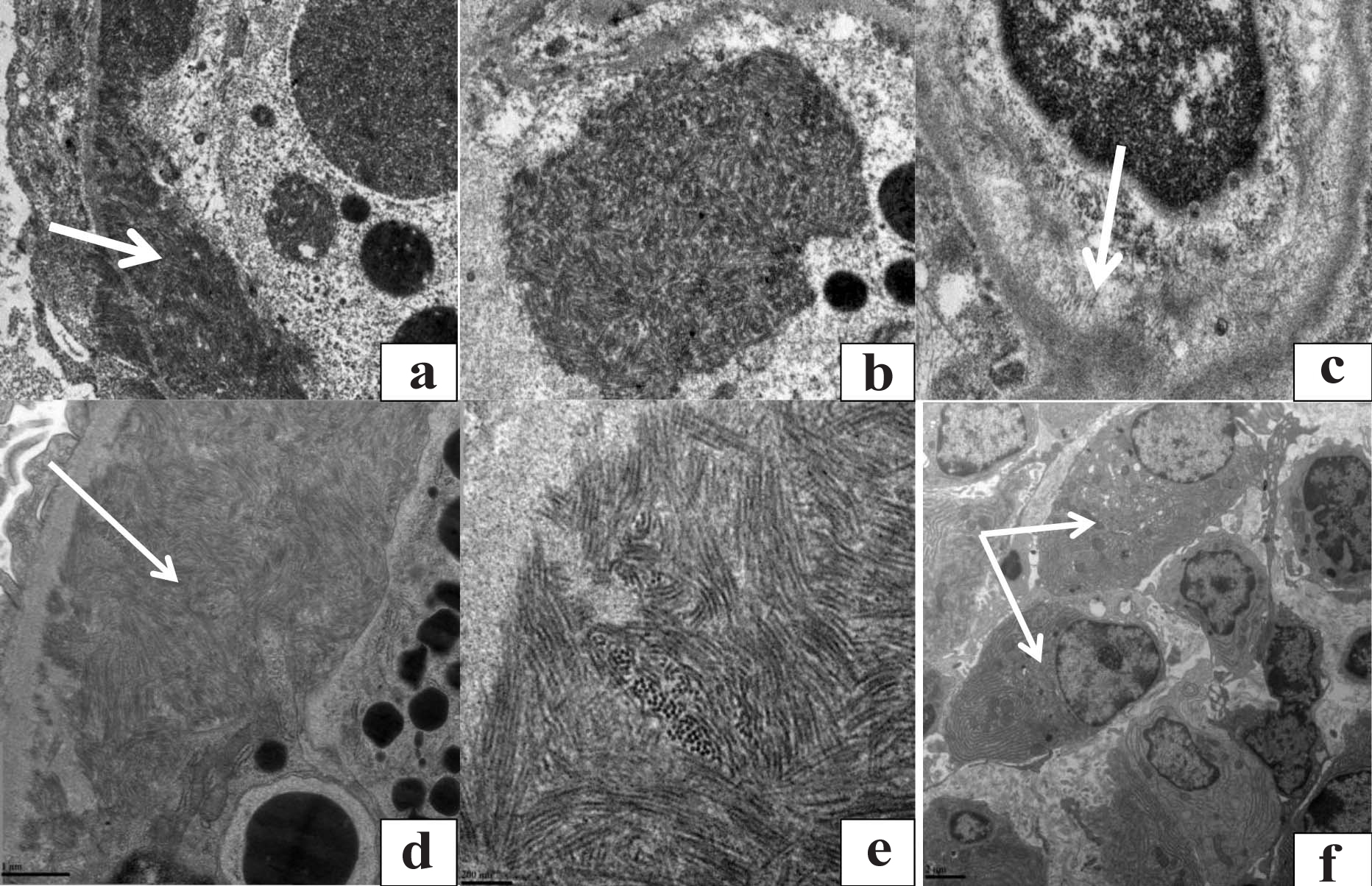
A kidney biopsy was performed and light microscopy revealed mesangio proliferative glomerulonephritis. No amyloid was found. The mesangium and glomerular capillary wall were positive for Ig G with an intensity of 3+ and only light-chain restriction for kappa was seen. Electron microscopy revealed glomeruli with electron-dense deposits located in the subendothelial space with less in the subepithelial space. These deposits were organised into microtubules (diameter 19 nm), forming tightly parallel arrays [Table/Fig-1a]. The capillary lumen was filled with swollen and dying cells. Clusters of 19-nm microtubules were detected in the cytoplasm of these cells [Table/Fig-1b].
Electron microscopy findings before treatment (a-c). Subendothelial and subepithelial microtubules, within the 19 nm range, were arranged in parallel arrays (a, x20500). Microtubules within cells located within the lumen of the capillary (b, x43000). An electron-transparent cavity formed from resorbed microtubules after treatment (c, 26500). Electron microscopy findings during a relapse (d-f). Large microtubular subendothelial deposits. (d, x40000). Ultrastructure of a microtubule: the pectoral cross is seen (e, x150000). Numerous plasma cells within the interstitial infiltrate (f, x12000).
The patient received eight courses of chemotherapy consisting of Bortezomib, Cyclophosphamide, and Dexamethasone (BorCyDex): a complete response was achieved after six cycles. Serum creatinine became normalised and macro-haematuria had stopped after the first round of chemotherapy.
Oedema and arterial hypertension resolved later. Glomerular-filtration rate, after eight courses of chemotherapy, was 90 mL/minute.
A repeat kidney biopsy was performed (after eight courses of chemotherapy), which showed segmental mesangial expansion, thickening of the segmental glomerular-basement membrane, and segmental sclerosis remained in the glomeruli. Immunofluorescent microscopy found no positivity for IgG or light-chain restriction for kappa. Electron microscopy showed electron-transparent cavities in the glomerular-basement membrane. Microtubules were absent [Table/Fig-1c].
The patient received autologous peripheral blood stem-cell transplantation in May 2010. However, an immunochemical relapse developed after two years (in May 2012) with an increase in paraprotein Gκ to the initial value of 11.7gm/L by November 2013. A clinical relapse was diagnosed in October 2015. Anaemia (Hb 9.9 gm/dL) and thrombocytopenia (73,000 μL) were revealed. Leukocytes (14,500/1mL), erythrocytes (28,000/1mL), and proteinuria (4.8 gm/24 hours) were found in the urinalysis. Glomerular-filtration rate remained normal (90 mL/minute). The bone marrow contained 30% clonal plasma cells. Fluorescence insitu hybridization showed deletion of 13q14. Osteolytic lesions were not found using whole-bone computed tomography. A repeat biopsy was performed. The glomeruli had increased in size and were abundantly infiltrated with lymphoid cells. The glomeruli had a lobular appearance caused by mesangial and endocapillary proliferation. Thickening of the glomerular basement membrane was revealed. There were deposits in the mesangium and into the glomerular basement membrane. Interstitial sclerosis was revealed in 25%-30%. The foci of lymphoid infiltration were within the interstitium. IgG(3+), IgA(+), IgM(+), light chains of κ-type(3+), and light chains of negative λ-type were seen using immunofluorescence. Electron microscopy revealed subendothelial deposits organised into packed microtubules (within the 11-12 nm range) [Table/Fig-1d,e]. Capillaries were abundantly infiltrated with lymphoid cells, some of which were plasma cells. Some capillary lumens were totally obstructed by these cells. Multiple high electron-dense protein inclusions were seen in all lymphoid cells. Interstitial infiltration was mainly from plasma cells [Table/Fig-1f]. The patient re-started treatment with BorCyDex.
Discussion
ITG is an unusual kidney lesion in MM, and is unlike cast-nephropathy. Remission of ITG with resorption of microtubular deposits can occur after effective treatment of MM. In our case, a relapse in MM was accompanied by a recurrence of ITG. In our opinion, complete remission is essential for resorption of ITG deposits. It should be noted that sustained complete remission from ITG in chronic lymphocytic leukaemia has been also described to occur soon after complete haematological remission was achieved [3,4].
Relapse of MM occurred two years after autologous peripheral blood stem-cell transplantation. A complete clinical picture of ITG was seen two years after the recurrence of a monoclonal secretion. Thus, ITG is a kidney lesion that develops slowly compared to cast nephropathy.
The pathogenesis of ITG remains unclear. In haematological diseases, ITG occurs predominantly in chronic lymphocytic leukaemia and is associated with paraproteinemia G [1,3]. However, secretion of paraprotein G is apparently not the main pathogenetic factor for the formation and deposition of microtubular deposits in the glomerular basement membrane. Monoclonal IgG secretion is present in only 11% cases of chronic lymphocytic leukaemia, while in MM it is present in 60% of patients [5,6]. However, ITG predominantly occurs in chronic lymphocytic leukaemia and is extremely rare in MM.
In this case report, we report some unusual features found in the electron-microscopy images of kidney-biopsy samples. The glomerular capillary lumen was filled, and in some places even obstructed by lymphoid cells. Accumulation of lymphoid and plasma cells within the glomerular-capillary lumen is, apparently, a special type of kidney-tumour lesion. Distinct signs of lymphoid-cell plasmatisation were noted: bundles of microtubules, similar to the deposits in kidneys, were revealed in the cytoplasm of some cells. It is likely that the microtubules were synthesised and formed in the lymphocytes and plasma cells that were present in the glomerular-capillary lumen, and then migrated to the glomerular basement membrane, leading to the development of ITG. It should be noted that tumour infiltration into the kidney in MM is very rare (1% of the cases) and, if it occurs, is usually found in the kidney interstitium [2,7].
Conclusion
This case presented with massive glomerular infiltration of plasma cells, which caused lumen obturation and interstitial infiltration, showing evidence for this specific kidney lesion. Bundles of microtubules, similar to the deposits in kidneys, were revealed in the cytoplasm of lymphoid and plasma cells. Effective antitumor therapy can lead to complete resorption of the microtubular deposits and result in complete recovery of kidney function.
[1]. Nasr SH, Fidler ME, Cornell LD, Leung N, Cosio FG, Sheikh SS, Immunotactoid glomerulopathy: clinicopathologic and proteomic studyNephrol Dial Transplant 2012 27(11):4137-46.10.1093/ndt/gfs34822872726 [Google Scholar] [CrossRef] [PubMed]
[2]. Xu W, Wang YH, Fan L, Fang C, Zhu DX, Wang DM, Prognostic significance of serum immunoglobulin paraprotein in patients with chronic lymphocytic leukemiaLeuk Res 2011 35(8):1060-65.10.1016/j.leukres.2010.12.00521208658 [Google Scholar] [CrossRef] [PubMed]
[3]. Bridoux F, Hugue V, Coldefy O, Goujon JM, Bauwens M, Sechet A, Fibrillary glomerulonephritis and immunotactoid (microtubular) glomerulopathy are associated with distinct immunologic featuresKidney Int 2002 62(5):1764-75.10.1046/j.1523-1755.2002.00628.x12371978 [Google Scholar] [CrossRef] [PubMed]
[4]. Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Bladé J, International staging system for multiple myelomaJ Clin Oncol 2005 23(15):3412-20.10.1200/JCO.2005.04.24215809451 [Google Scholar] [CrossRef] [PubMed]
[5]. Nasr SH, Valeri AM, Sethi S, Fidler ME, Cornell LD, Gertz MA, Clinicopathologic correlations in multiple myeloma: A case series of 190 patients with kidney biopsiesAm J Kidney Dis 2012 59(6):786-94.10.1053/j.ajkd.2011.12.02822417785 [Google Scholar] [CrossRef] [PubMed]
[6]. Castro JE, Diaz-Perez JA, Barajas-Gamboa JS, Horton JM, Weidner N, Kipps TJ, Chronic lymphocytic leukemia associated with immunotactoid glomerulopathy: a case report of successful treatment with high-dose methylprednisolone in combination with rituximab followed by alemtuzumabLeuk Lymphoma 2012 53(9):1835-38.10.3109/10428194.2012.66391422335532 [Google Scholar] [CrossRef] [PubMed]
[7]. Nasr SH, Alobeid BB, Otrakji JA, Markowitz GS, Myeloma cast nephropathy, direct renal infiltration by myeloma, and renal extramedullary haematopoiesisKidney Int 2008 73(4):517-18.10.1038/sj.ki.500241618235533 [Google Scholar] [CrossRef] [PubMed]