The axillary brachial plexus block was first described by William Stewart Halstead at St. Luke’s Roosevelt Hospital Center, New York City in 1884. It is a commonly used regional anaesthetic technique for elbow, forearm and hand surgery and popular because of its ease, reliability and safety. This route avoids the potential complications like diaphragmatic paresis, pneumothorax and cervical sympathetic nerve blockade associated with the other routes of brachial plexus block [1]. In animal studies, dexamethasone has been shown as a non-particulate steroid that is cytoprotective and non-neurotoxic [2,3]. Although, the exact mechanism of action of dexamethasone as a local anaesthetic adjuvant in regional anaesthesia is unknown, studies suggest that dexamethasone may prolong block duration by increasing the activity of inhibitory potassium channels on nociceptive C fibers [4]. Dexamethasone causes vasoconstriction via glucocorticoid receptor mediated nuclear transcription modulation and suppresses inflammatory mediators and Prostaglandins (PGE2) [5]. Non-Steroidal Anti-Inflammatory Drugs (NSAID) inhibit synthesis of prostaglandins from arachidonic acid in phospholipid membranes resulting in decreased afferent nociceptive signals from the site of surgery. Various clinical studies have supported an enhanced analgesic effect when NSAIDs are concentrated at a peripheral site compared to the systemic administration thereby suggesting a predominantly peripheral site of action [6,7]. Ketorolac is a parenteral NSAIDs. Studies have shown that ketorolac as an adjuvant to local anaesthetics during peripheral nerve block enhanced duration and quality of analgesia [8,9]. The advantage of ultrasound guidance in performing regional blocks is a high success rate and safety, direct visualisation of neuronal and adjacent structures and spread of local anaesthetics and reducing the incidence of inadvertent intravascular or intra neural injections. The aim of our study was to provide a safe and cost-effective method of post-operative analgesia with reduced need for systemic analgesics and early ambulation for patients with isolated hand and forearm injuries. The primary aim of our study was to compare the duration of post-operative analgesia in axillary brachial plexus blocks with ketorolac versus dexamethasone as an adjuvant to bupivacaine. The secondary aim was to study the effect of these adjuvants on sensory and motor block characteristics.
Materials and Methods
This prospective randomised double-blinded study was done at Saveetha Medical College and Hospital, Chennai from March 2016 to January 2017. After obtaining Institutional Ethical Committee clearance (EC NO-027/03/2016/IEC/SU) and patient’s written informed consent, this study was conducted on 120 patients between 20 to 60 years of age belonging to American Society of Anaesthesiologists (ASA) I or II status, scheduled for isolated hand and forearm surgeries. This study was registered with Clinical trial registry India with reference number: CTRI/2017/02/007915. Exclusion criteria was patients with suspected coagulopathy, neuropathies, known allergies to local anaesthetic drugs or adjuvants and pregnant and lactating women. Sample size was estimated using time to first rescue analgesic requirement among three groups as the main primary variable. A pilot study was conducted on 15 patients and to detect a projected difference of 20% in duration of post-operative analgesia between the groups, with a type I (α) error of 0.05 and a power of 0.90, the pilot data had demonstrated that the mean time to first rescue analgesic was 600±170 minutes, 1000±180 minutes and 850±160 minutes for Groups C, D and K respectively. To detect a clinical difference with a standard deviation of 150 minutes, it was calculated that 38 subjects allocated to each group would provide a power of 90% at a type I error rate of 0.05. However, we assigned 40 patients to each group. The enrolled patients were divided randomly into three groups [Group C (n=40), Group D (n=40) and Group K (n=40)] using computer generated randomisation. The assigned random group was enclosed in a sealed envelope to ensure concealment of allocation sequence.
The drug solutions were prepared by an anaesthesiologist not involved in the study and another anaesthesiologist who was blinded performed the axillary block. The data collection was done by a blinded observer. The patients enrolled in the study were examined pre-operatively and the procedure was explained to each patient. In the operating room the patients were connected to a multipara monitor and heart rate, ECG, non-invasive blood pressure and peripheral oxygen saturation were monitored. An Intravenous (IV) cannula was inserted in the adjacent non operative hand and IV infusion of Hartmann’s solution was started. IV midazolam 0.03–0.04 mg/kg was given and oxygen was administered at 4 L/min through a Hudson mask. The patients enrolled in the study were positioned supine with the arm abducted, forearm supinated and elbow flexed with the hand either behind or above the head. Under sterile precautions cleaning and draping of the axilla was done. The block was performed using a portable digital colour doppler ultrasound system (Sonoscape model: S8 Exp, Europe S.r.l.) with a high frequency linear array probe (8–13 MHz). The transducer probe was placed transversely on the proximal, medial upper arm. After the equipment was set the skin was anaesthetised with 2 mL of 2% lignocaine prior to introducing the block needle. Patients in Group C (n=40) received 30 mL of 0.375% bupivacaine plus 2 mL of 0.9% normal saline, Group D (n=40) received 30 mL of 0.375% bupivacaine plus 2 mL of dexamethasone (8 mg) and Group K (n=40) received 30 mL of 0.375% bupivacaine plus 1 mL of ketorolac (30 mg) in 1 mL of 0.9% normal saline around the brachial plexus through the axillary route. The in-plane technique was used for better needle visualisation and needle accuracy. After visualisation of the pulsatile axillary artery, the radial, median and ulnar nerves were identified and blocked separately. The musculocutaneous nerve located either between the biceps and coracobrachialis or within the body of the coracobrachialis was blocked separately. Inadvertent intravascular injection was avoided by frequent aspiration and visualisation on the screen of the ultrasound machine. Inadvertent intraneural injections were also avoided by stopping the injection when the patient complained of pain during injection. The onset, peak and duration of motor block and sensory block, time to first rescue analgesic, pain scoring by Visual Analogue Scale (VAS), heart rate and blood pressure were recorded for each patient. Sensory and motor blockade were assessed at every two minutes interval after completion of injection until peak action and then after the end of surgery until first 24 hours till the block had completely worn off. Heart rate and blood pressure was noted at every ten minutes interval throughout surgery. The patients were monitored in the post anaesthesia care unit for the first 24 hours. Assessment of sensory block was done in the dermatomal areas corresponding to the median nerve, radial nerve, ulnar nerve and musculocutaneous nerve till complete sensory blockade was achieved. Following completion of the local anaesthetic injection, the sensory block was evaluated by Hollmen scale as: 1-normal sensation of pinprick; 2-pinprick felt as sharp pointed but weaker compared with same area in the other upper limb; 3-pinprick recognised as touch with blunt object and 4-no perception of pinprick. The onset time of the sensory block was taken as the time interval in minutes from injection of the local anaesthetic till a Hollmen score of 2, while the time for the complete sensory block was taken from injection of the local anaesthetic till a Hollmen score of 4. The total duration of the sensory block was taken as the duration of the time in minutes from the time of complete sensory block till the time when the Hollmen score less than 4 was reached. Motor block was monitored by thumb adduction (ulnar nerve), thumb abduction (radial nerve), thumb opposition (median nerve) and flexion of elbow and pronation of forearm (musculocutaneous nerve) using a modified Bromage Scale for the upper extremity; 0-normal motor function with full flexion and extension of elbow, wrist, and fingers; 1-decreased motor strength with ability to move the fingers only; 2-complete motor block with inability to move the fingers and 3-unable to move the arm, elbow or fingers. Onset of motor blockade was considered as the time interval in minutes from injection of the local anaesthetic till when there was Grade-1 motor blockade. Peak motor block was considered as the time interval in minutes from injection of the local anaesthetic till when there was Grade-3 motor blockade. The total duration of the motor block was taken as the duration of the time in minutes between the times to complete motor block till normal motor function (Grade-0). Pain was assessed by using the VAS in which a score of 0 indicates no pain and a score of 10 worst pain. The VAS measurements were obtained every three hours post-operatively at 3,6,9,12,15,18,21 and 24 hours. Rescue analgesic in the form of slow IV bolus of 50 mg of tramadol was administered at the VAS score of 4. Time of first rescue analgesic and the total analgesic given to each patient during the first 24 hours post-operative period were recorded.
Statistical Analysis
Statistical analysis was performed by SPSS (version 18.0, IBM Co. Chicago, Illinois, USA). Chi-square analysis was used to compare the qualitative variables. For the analysis of quantitative variables student’s paired t-test was used. Pain levels according to the VAS were analysed by the Friedman test, with intergroup comparison by the Kruskall-Wallis test followed by the Dunn test in case of difference among the groups. A p-value<0.05 was considered as statistically significant and a p<0.001 as highly significant. Interval data were expressed as mean and standard deviation.
Results
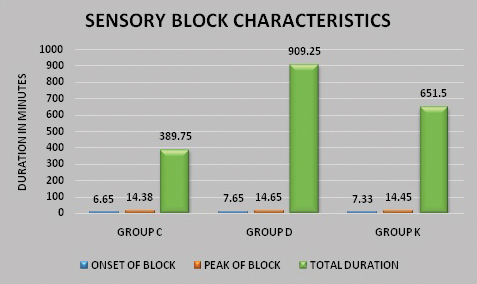
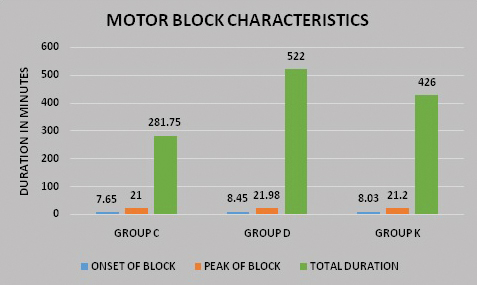
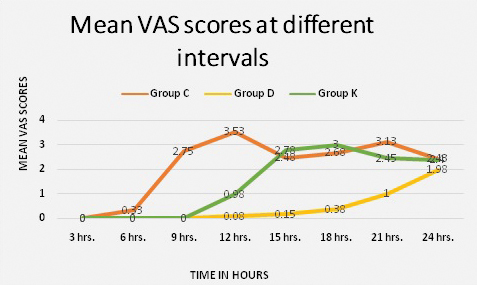
A total of 132 patients with isolated hand and forearm injuries were randomised into three groups. Four patients with failed block and eight patients with patchy block were excluded from the study as shown in the consort diagram [Table/Fig-1]. Demographic parameters such as age wise and sex wise distribution of the participants were comparable in the three groups. There was however, a larger number of males compared to females in each group [Table/Fig-2]. The block characteristics are shown in [Table/Fig-3]. The mean duration of surgery was 48.13±21.53 minutes in Group C, 82.13±38.22 minutes in Group D and 71.25±30.60 minutes in Group K (p<0.001). This was statistically significant but clinically not relevant as it had no effect on the outcomes of the study. The onset of sensory block was earlier in Group C (6.65±0.97 minutes) as compared to Group D (7.65±0.83 minutes) and group K (7.33±0.97 minutes); the p-value between Groups C and D and Groups C and K was significant (p-value<0.001) as shown in [Table/Fig-4]. The minimal delay in onset of sensory block in Groups D and K compared to Group C was not clinically relevant though statistically significant [Table/Fig-4]. The peak of sensory block was comparable in the three groups; Group C (14.38±1.78 minutes) compared to Group D (14.65±1.46 minutes) and Group K (14.45±1.75 minutes). The total duration of sensory block was 389.75±40.35 minutes in Group C, 909.25±76.74 minutes in Group D and 651.50±75.77 minutes in Group K as shown in [Table/Fig-4]; the p-value between Groups C, D and K was significant (p<0.001). The onset of motor block was earlier in Group C (7.65±0.86 minutes) as compared to Group D (8.45±0.78 minutes) and Group K (8.03±0.89 minutes); the p-value between Groups C and D and Groups C and K was significant (p<0.001). The delay in onset of motor block in Groups D and K compared to Group C was not clinically relevant though statistically significant. The peak of motor block was comparable in Group C (21.00±2.15 minutes), Group D (21.98±1.49 minutes) and Group K (21.20±1.56 minutes). The total duration of motor block was 281.75±38.68 minutes in Group C, 522.00±53.74 minutes in Group D and 426.00±60.07 minutes in Group K (p<0.001) as shown in [Table/Fig-4,5]. The onset of pain was much earlier in Group C, the mean VAS score was three at ninth post-operative hour whereas it was zero at the ninth post-operative hour in Group D and Group K (p<0.001) [Table/Fig-6]. In Group D, out of 40 patients only eight received rescue analgesic in the first 24 hours. Post-operative distribution of patients according to VAS score of four showed that all patients in Group C required rescue analgesic by nine hours and in Group K all patients received rescue analgesic by 15 hours. In Group D only eight patients received rescue analgesic and only after 18 hours post-operatively. The average dose of rescue analgesic received in the first 24 hours was 11.25±21.15 mg in Group D, 98.75±21.15 mg in Group C and 61.25±23.99 mg in Group K (p<0.001). The magnitude of heart rate and mean arterial pressure changes between the groups were comparable [Table/Fig-7,8]. There were no adverse effects or complications related to the procedure or the drugs used in any of the patients enrolled in our study.
Consort diagram showing flow of participants.
Demographic data and duration of surgery.
| Parameters | Group C(Mean±SD)(n=40) | Group D(Mean±SD)(n=40) | Group K(Mean±SD)(n=40) | p-value |
|---|
| 1. Age (years) | 30.48±10.85 | 27.03±10.01 | 28.93±10.88 | 0.348 |
| 2. Male/Female | 35/5 | 34/6 | 32/8 | 0.646 |
| 3. Duration of surgery (minutes) | 48.13±21.53 | 82.13±38.22 | 71.25±30.60 | p<0.001*** |
SD: Standard deviation, Group C (control), Group D (dexamethasone), Group K (ketorolac)
Sensory block characteristics.
Comparison of block characteristics.
| Parameters | Group C | Group D | Group K | p-value | p-value between groups |
|---|
| Mean±SD | Mean±SD | Mean±SD |
|---|
| Sensory block onset (minutes) | 6.65±0.97 | 7.65±0.83 | 7.33±0.97 | p<0.001*** | Group C and D-0.000***Group C and K-0.000***Group D and K-0.265 (NS) |
| Peak of sensory block (minutes) | 14.38±1.78 | 14.65±1.46 | 14.45±1.75 | p>0.05 | NS |
| Total duration of sensory block (minutes) | 389.75±40.35 | 909.25±76.74 | 651.50±75.77 | p<0.001*** | Group C and D-0.000***Group C and K-0.000***Group D and K-0.000*** |
| Motor block onset (minutes) | 7.65±0.86 | 8.45±0.78 | 8.03±0.89 | p<0.001*** | Group C and D-0.000***Group C and K-0.000***Group D and K-0.068 (NS) |
| Peak of motor block (minutes) | 21.00±2.15 | 21.98±1.49 | 21.20±1.56 | p>0.05 | NS |
| Total duration of motor block (minutes) | 281.75±38.68 | 522.00±53.74 | 426.00±60.07 | p<0.001*** | Group C and D-0.000***Group C and K-0.000***Group D and K-0.000*** |
| Time to rescue analgesic tramadol (minutes) | 570.00±197.37 | 1260.0±184.93 | 964.61±190.41 | p<0.001*** | Group C and D-0.000***Group C and K-0.000***Group D and K-0.000*** |
| Total dose of analgesic (tramadol) in mgs in 24 hours. | 98.75±21.15 | 11.25±21.15 | 61.25±23.99 | p<0.001*** | Group C and D-0.000***Group C and K-0.000***Group D and K-0.000*** |
SD: Standard deviation, Group C (control), Group D (dexamethasone), Group K (ketorolac)
***p<0.001 (0.000-0.001)-highly significant, **p<0.01 (0.002-0.01)-very significant, *p<0.05 (0.02-0.05)-significant, (NS) p>0.05-not significant
Motor block characteristics.
Pain scores in the three groups.
Changes in heart rate in the three groups.
Changes in mean arterial pressure in the three groups.
Discussion
Dexamethasone has been proved as an adjuvant to local anaesthetic in axillary brachial plexus blocks and other regional plexus blocks. However, the use of ketorolac as an adjuvant to local anaesthetic in brachial plexus blocks is supported by very few studies [6,7]. The purpose of this study was to assess the efficacy of dexamethasone and ketorolac as two different types of local anaesthetic adjuvants in ultrasound guided axillary brachial plexus block. Corticosteroids are not licensed for perineural use in USA and Europe but they still continue to be used as adjuvants to local anaesthetics in regional nerve blocks. Though, neurotoxicity may be a potential concern with any adjuvant, data suggests that dexamethasone attenuates local anaesthetic induced nerve toxicity [5,9]. Dexamethasone as an adjuvant to bupivacaine has shown beneficial effects on block duration without long-term evidence of neurotoxicity and an attenuation of perineural inflammatory responses generated by local anaesthetics [8,9]. Bagriyanik HA et al., and Hseih YC et al., investigated the effects of intrathecal ketorolac on spinal cord-traumatised rats and demonstrated its neuro protective action [10,11]. Cinar O et al., demonstrated that intraperitoneal administration of ketorolac in rats with induced sciatic nerve injury promoted early nerve regeneration and functional recovery [12]. Eisenach JC et al., in his two studies reported that intrathecal administration of ketorolac did not have any neurotoxic effects in humans [13,14].
In ongoing infections, immune compromised states and diabetes where steroids are contraindicated, NSAIDs can be used. Hence, we considered comparison of a steroid with an NSAID. In our study, the duration of post-operative analgesia was significantly longer in Group D (1260.0±184.93 minutes) than Group K (964.61±190.41 minutes) and Group C (570.00±0.197.37 minutes). Hence, dexamethasone showed a promising increase in post-operative analgesia which was consistent with supporting studies [15-20]. Our study shows that, the patients in control Group C received rescue analgesic much earlier than Group K and Group D. The results about the delayed time to analgesic request by the use of adjuvant drugs (dexamethasone and ketorolac) support the previous studies [6,7,15-20]. Our study showed that, adjuvant dexamethasone had better effects on sensory and motor block duration than ketorolac and the control group. In our study, the minimum delay in the onset of sensory and motor block in Groups D and K compared to Group C could be because of lowering of the pH of the mixture by addition of adjuvants. The delay in onset of block by dexamethasone is consistent with the study by Knezevic NN et al., [21]. However, the study done by Movofegh A et al., showed that dexamethasone had no effect on the onset of block and Biradar PA et al., showed that dexamethasone enhanced the onset of action of local anaesthetic [18,20]. In our study, the total duration of motor and sensory block was significantly longer in dexamethasone and ketorolac groups compared to control group (p<0.001). There are studies supporting ketorolac as adjuvant to local anaesthetic in Intra Venous Regional Anaesthesia (IVRA) and other peripheral nerve blocks [22-24], but very few studies in brachial plexus blocks [6,7].
There are no studies in literature comparing dexamethasone with ketorolac as adjuvants to local anaesthetic in brachial plexus blocks. Our results about ketorolac as an adjuvant to bupivacaine on the duration of sensory and motor block and post-operative analgesia supported the study by Mirkheshti A et al., and Basenko IL et al., [6,7]. In our study, we chose a concentration of 0.375% bupivacaine to use an effective volume without causing toxicity. This is supported by the study claiming that the mass of bupivacaine rather than the concentration is the major determinant of the ED50 for achieving a successful block [25].
Limitation
In this study peri-operative blood glucose concentration was not evaluated in the dexamethasone group and the patients were followed up only for two weeks for assessing nerve injuries.
Conclusion
Adding 30 mg ketorolac or 8 mg dexamethasone to 30 mL bupivacaine 0.375% for axillary brachial plexus block effectively prolongs the duration of post-operative analgesia and the duration of motor and sensory block without any effect on the heart rate and blood pressure.
These effects are more enhanced with dexamethasone than ketorolac. There is however, a minimal delay in onset of block when these adjuvants are used with 0.375% bupivacaine.
SD: Standard deviation, Group C (control), Group D (dexamethasone), Group K (ketorolac)
SD: Standard deviation, Group C (control), Group D (dexamethasone), Group K (ketorolac)
***p<0.001 (0.000-0.001)-highly significant, **p<0.01 (0.002-0.01)-very significant, *p<0.05 (0.02-0.05)-significant, (NS) p>0.05-not significant