Introduction
E-cadherin is a glycoprotein cell adhesion molecule, which is important for maintenance of epithelial integrity and its loss is a useful biomarker for diagnosis and prognosis of preneoplastic and neoplastic squamous cell lesions.
Aim
To evaluate and compare the expression of E-cadherin in oral and cervical premalignant and malignant squamous cell lesions.
Materials and Methods
A total of 66 specimens (nine normal, 32 premalignant and 25 malignant) were obtained from oral cavity and cervix including 41 female and 25 male patients, which were divided into Group-I (control), Group-II (premalignant) and Group-III (malignant). Tumour grading and staging was performed. Immunohistochemical staining was done using monoclonal mouse antibody, E-cadherin as per manufacturer’s instruction. Analysis was performed using SPSS software version 17.0.
Results
E-cadherin expression showed significantly (p=0.006) higher grades of expression in Group-I as compared to Group II and group III. Expression of E-cadherin was homogenous with membranous pattern in 100% cases of group I and was found in basal and parabasal layers of epithelium. Among group III, well-differentiated squamous cell carcinoma showed strong staining (++) in 92.3% of cases as compared to moderately/poorly differentiated tumours (16.7% of cases). As tumour become poorly differentiated, cytoplasmic staining was predominant pattern in 50% of cases of moderately/poorly differentiated tumour. Overall comparison of E-cadherin expression between oral and cervix lesions showed insignificant difference (p>0.05).
Conclusion
E-cadherin expression was found to inversely correlate with the loss of cell differentiation. It can be used as prognostic marker for both oral and cervical premalignant and malignant squamous cell lesions.
Cervix, Squamous cell carcinoma, Tumour
Introduction
Oral Squamous Cell Carcinoma (SCC) is the most prevalent malignancy in oral cavity. It is the sixth most common malignancy in the world and ranks as first in males in the Indian subcontinent [1,2]. Cervical cancer (squamous cell carcinoma) is one of the most common malignant gynaecological tumours and the third leading cause of cancer death in women [3].
Both oral and cervical SCC constitutes a major cause of cancer morbidity and mortality in males and females. In spite of the advances made in surgical treatment, adjunctive therapy and radiotherapy; the survival rate in cervical and oral SCC remains unchanged in past three decades, thereby making these neoplasms a major health problem worldwide [3-5]. Understanding molecular mechanisms regulating SCC progression is a prerequisite for improving the patient prognosis and management.
Cell Adhesion Molecules (CAMs) play a central role in pathogenesis and progression of malignant tumours. Cells are connected to each other by tight cell to cell adhesion mediated by a family of glycoproteins named cadherins. In epithelial cells, the adhesiveness is mediated by epithelial cadherins (E-cadherins), a 120 Kd transmembrane glycoprotein localised in cell junctions [1,6]. Many authors favour the theory of Epithelial Mesenchymal Transition (EMT) to explain tumour progression.
As carcinoma progresses to invasive tumour, it loses the epithelial morphology and acquires the mesenchymal cell like phenotype, called as EMT. This change enhances migratory, invasive and metastatic behaviours of carcinoma cells, and yields chemo-resistance and stem cell-like features [7,8]. Loss of E-cadherin expression has been correlated with a high grade and an advanced stage of cancer, with poor prognosis in oesophageal [9], gastric [10], colon [11], breast [12], lung [13], pancreatic [14], squamous head and neck tumours [15], and uterine cervix tumours [16]. In addition, alteration of E-cadherin expression has also been reported in preinvasive lesions including oral lesions such as leukoplakia and cervical intraepithelial neoplasm [17,18]. Therefore, E-cadherin dependent cell to cell adhesion is important for maintenance of epithelial integrity and its loss is a useful biomarker to prognosticate for preneoplastic and neoplastic lesions. The purpose of this study was to evaluate and compare the expression of E-cadherin in oral and cervical premalignant and malignant squamous cell lesions to elucidate its role as a reliable and potential biomarker.
Materials and Methods
This observational study was conducted prospectively for one-year duration in the Department of Pathology, King George’s Medical University, Lucknow, India. A total of 66 specimens were obtained from oral cavity and cervix comprising of 41 females and 25 males with patient’s age ranging from 30-70 years. The relevant clinical and demographic details were collected. Sample was distributed into three groups: Group-I (control- hypertrophied/normal oral and cervical mucosa), Group-II (premalignant) and Group-III (malignant) as shown in [Table/Fig-1]. Premalignant group comprised of nine leukoplakia and eight moderate to severe oral epithelial dysplasia and 15 cases from cervix including different grades of Cervical Intraepithelial Neoplasia (CIN).
Sample distribution in various groups.
| Group | No. of specimens(N=66) | Cervical(N=32) | Oral(N=34) |
|---|
| N | % | N | % |
|---|
| Group-I (Control) | 9 | 5 | 15.60 | 4 | 11.80 |
| Group-II (Premalignant) | 32 | 15 | 46.90 | 17 | 50.00 |
| Group-III (Malignant) | 25 | 12 | 37.50 | 13 | 38.20 |
χ2=0.216 (df=2), p=0.898
Three to four micron thin sections were obtained from formalin fixed embedded tissue blocks of each specimen. Histopathological examinations were done on Hematoxylin and Eosin (H&E) stained sections. Tumour grading was done according to Broders classification (1920) on the basis of differentiation and keratinization of tumour cells [19]. Tumour staging was carried out according to Tumour, Nodes, Metastases (TNM) classification and oral epithelial neoplasia was classified according to World Health Organization (WHO) classification (2008) [20].
For Immunohistochemistry (IHC), all the study sections were stained and evaluated by using monoclonal mouse antibody; E-cadherin (manufactured by Dako Flex, clone NCH-38, ready to use) as per standard protocol. Interpretation was done according to the criteria defined by Kaur G et al., [21]. Quantitative and qualitative criteria was defined as follows.
Quantitative criteria: Staining was absent (score 0), Heterogeneous staining (score +/0), weak staining (score +) and strong staining (score ++).
Qualitative criteria: It defines the site of staining in the cell. Absence of staining (1), Membranous staining (2), Both membranous and cytoplasmic staining (3), Cytoplasmic staining (4).
Statistical Analysis
Data analysis was performed using SPSS software (window version 17.0). The data were summarised as number (N), percentages (%) and mean±SD (standard deviation) for each group. Groups were compared by student’s t-test, Kruskal-Wallis H test, Mann-Whitney U test and Chi-square test. p-value<0.05 was considered as statistically significant.
Results
Maximum number of subjects in premalignant group was aged between 41-50 years while in malignant group aged between 51-60 years. Among the oral SCC, the most common site was buccal mucosa. Quantitative assessment of E-Cadherin expression showed significantly (p=0.006) higher grades of expression in Group-I (100%) as compared to Group II (87.5%) and III (56%) and statistically significant difference was found between (control) Group -I vs (malignant) Group III and (premalignant) Group II vs (malignant) Group III [Table/Fig-2,3]. Among the malignant group well-differentiated SCC showed strong staining (++) in 92.3% (12/13) of cases as compared to moderately/poorly differentiated tumours (16.7% (2/12) of cases) [Table/Fig-4].
Distribution of subjects according to quantitative assessment of E-cadherin expression.
| S.No. | Score | TotalN=66 | Group I(Control)N=9 | Group II(Premalignant)N=32 | Group III(Malignant)N=25 |
|---|
| N | % | N | % | N | % |
|---|
| 1. | 0 | 1 | 0.00 | 0.00 | 0 | 0.00 | 1 | 4.00 |
| 2. | +/0 | 8 | 0.00 | 0.00 | 3 | 9.34 | 5 | 20.00 |
| 3. | + | 6 | 0.00 | 0.00 | 1 | 3.13 | 5 | 20.00 |
| 4. | ++ | 51 | 100.00 | 100.00 | 28 | 87.50 | 14 | 56.00 |
χ2=10.330 (df=2), p=0.006 (Kruskal-Wallis test)
(0 = Absence, +/0 = Heterogeneous, + = Weak, ++ = Strong)
Comparison of quantitative expression of E-cadherin in various groups (Mann-Whitney U test).
| S.No. | Comparison | Z | P |
|---|
| 1. | Group I vs Group II | 0.271 | 0.271 |
| 2. | Group I vs Group III | 2.335 | 0.020 |
| 3. | Group II vs Group III | 2.462 | 0.010 |
Association of quantitative expression of E-cadherin with degree of differentiation in Group III.
| S.No. | Score | N=25 | Well differentiated(N=13) | Moderately/Poorly differentiated(N=12) |
|---|
| N | % | N | % |
|---|
| 1. | 0 | 1 | 0 | 0.00 | 1 | 8.33 |
| 2. | +/0 | 5 | 0 | 0.00 | 5 | 41.67 |
| 3. | + | 5 | 1 | 7.69 | 4 | 33.33 |
| 4. | ++ | 14 | 12 | 92.30 | 2 | 16.67 |
z= 3.748, p<0.001
Qualitative expression of E-cadherin with degree of differentiation showed that as tumour became poorly differentiated, it lost the membranous pattern and localise as faint cytoplasmic staining (score-4 pattern) which was found as predominant pattern in 50% (6/12) of cases of moderately/poorly differentiated tumour [Table/Fig-5,6 and 7]. On intergroup comparison, statistically significant difference for E-cadherin expression was found between all the groups [Table/Fig-8].
Association of qualitative expression of E-cadherin with degree of differentiation in Group III.
| S.No. | Score | N=25 | Well differentiated(N=13) | Moderately/Poorly differentiated(N=12) |
|---|
| N | % | N | % |
|---|
| 1. | 1 | 1 | 0 | 0.00 | 1 | 8.33 |
| 2. | 2 | 1 | 1 | 7.69 | 0 | 0.00 |
| 3. | 3 | 15 | 10 | 76.92 | 5 | 41.67 |
| 4. | 4 | 8 | 2 | 15.39 | 6 | 50.00 |
z= 1.505, p=0.205 (1= Absent staining, 2= Membranous staining, 3= Membranous and Cytoplasmic staining, 4= cytoplasmic staining)
Distribution of subjects according to qualitative assessment of E-cadherin expression.
| S.No. | Score | TotalN=66 | Group I(Control)N=9 | Group II(Pre-malignant)N=32 | Group III(Malignant)N=25 |
|---|
| N | % | N | % | N | % |
|---|
| 1. | 1 | 1 | 0 | 0.00 | 0 | 0.00 | 1 | 4.00 |
| 2. | 2 | 15 | 9 | 100.00 | 5 | 15.62 | 1 | 4.00 |
| 3. | 3 | 41 | 0 | 0.00 | 26 | 81.26 | 15 | 60.00 |
| 4. | 4 | 9 | 0 | 0.00 | 1 | 3.13 | 8 | 32.00 |
χ2= 26.915 (df=2), p<0.001 (Kruskal-Wallis test)
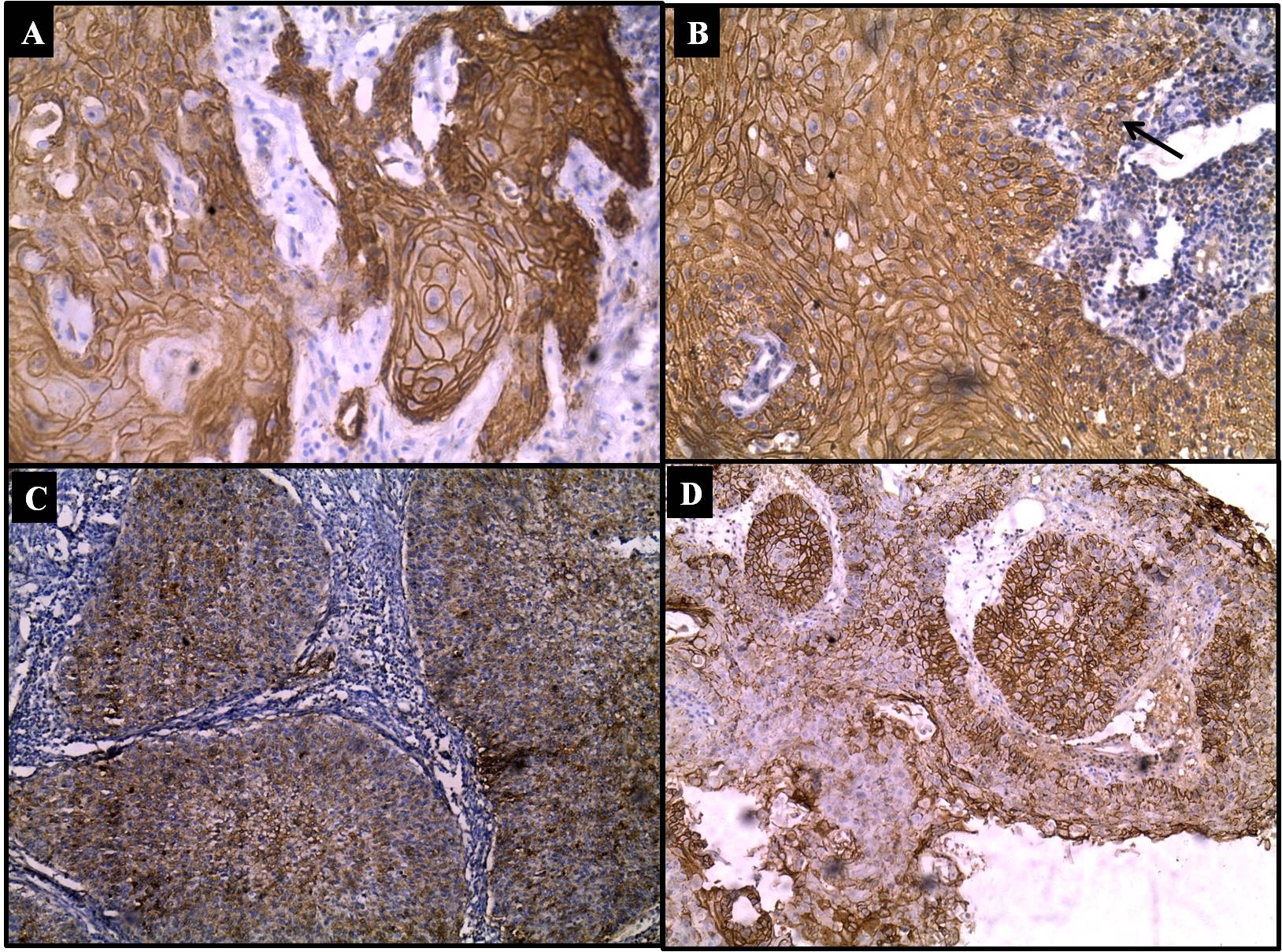
Immunostaining: E-cadherin expression in SCC; a) cytoplasmic and membranous staining in well differentiated SCC (IHC, 20X); b) cytoplasmic and membranous staining in micro invasive SCC (arrow invasive cluster) (IHC, 20X); c) cytoplasmic staining of E-cadherin in moderately differentiated SCC (IHC, 10X); d) heterogenous membranous and cytoplasmic staining of E-cadherin in moderately differentiated SCC (IHC, 10X).
Comparison of qualitative expression of E-cadherin in various groups (Mann-Whitney U test).
| S.No. | Comparison | z-value | p-value |
|---|
| 1. | Group I vs Group II | 4.556 | <0.001 |
| 2. | Group I vs Group III | 4.128 | <0.001 |
| 3. | Group II vs Group III | 2.537 | 0.011 |
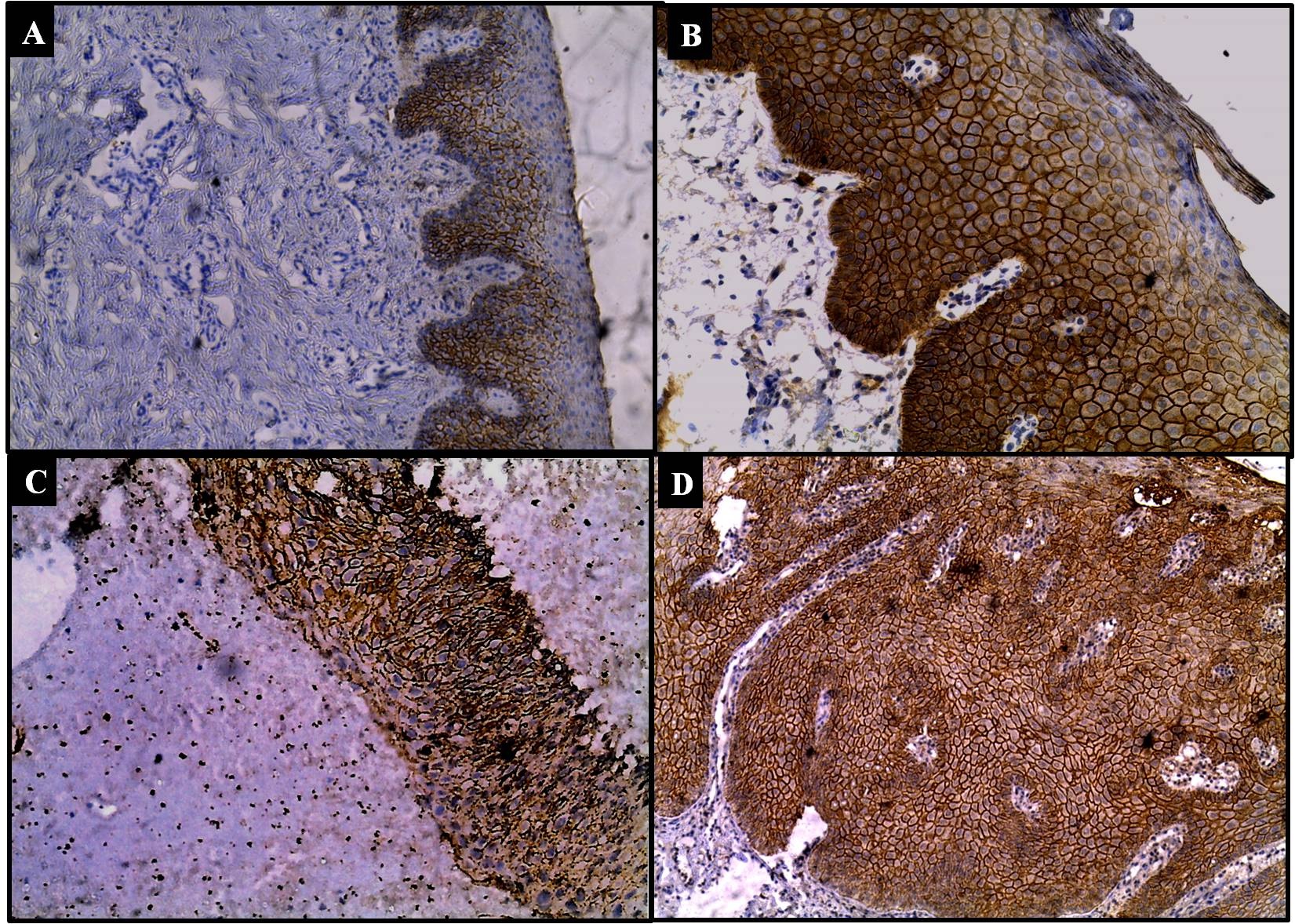
Expression of E-cadherin was found to be predominantly membranous 100% (9/9) in basal and parabasal layers of epithelium in control group while it is score two or three and expressed full thickness in dysplasia cases (group II) [Table/Fig-9]. Overall comparison of E-cadherin expression between oral and cervix lesions, showed no significant difference (p>0.05).
Immunostaining: E-cadherin expression in squamous premalignant lesions in oral and cervical mucosa; a) cytoplasmic and membranous staining in basal parabasal layers in normal epithelium (IHC, 10X); b) cytoplasmic and membranous staining in all layers in leukoplakia (IHC, 20X); c) cytoplasmic and membranous staining of full thickness epithelium in dysplasia (IHC, 10X); d) membranous and cytoplasmic staining of E-cadherin in full thickness epithelium in leukoplakia with dysplasia (IHC, 10X).
Discussion
The two most common epithelial malignancies in Indian sub-continent encountered in day to day reporting are oral carcinoma and cervical carcinoma. Epithelial malignancy has a particular pattern of cell arrangement and differentiation. It often progresses through dysplasia carcinoma in situ-invasive carcinoma sequence [1-5,15]. E-cadherin plays an important role in the histogenesis and the maintenance of the structural integrity of the normal epithelium. When a cell acquires malignant potential then it loses adhesion with surrounding cells with increased cellular motility and also loss of epithelial morphology [6-8,22]. E-cadherin is a tumour suppressor and its altered level plays a significant role in the advanced stages of carcinoma progression due to its ability to integrate cell to cell adhesion with growth signalling [7,8,23]. Loss of adhesion or reduction of this protein staining can be caused by deletion or mutation silencing by CpG methylation or by altered gene expression of E-cadherin but the exact mechanism is yet to be explored [21,24].
In present study, we observed that in surface epithelium of healthy oral mucosa and cervix (Group-I), expression of E-cadherin was strong and homogenous. The immunostaining pattern was membranous and confined to basal and parabasal layers excluding the uppermost epithelial layer. Whereas, in premalignant conditions (leukoplakia and dysplasia -Group-II) showed strong positive staining of membranous and cytoplasmic pattern covering full thickness of squamous epithelium in majority of patients (81.3%). However, in severe dysplasia cases it becomes more cytoplasmic and lost in basal layers. Similar, results were observed by Yogesh TL et al., and Monal B. Yuwanati et al., [25,26] and they conclude that the reduced expression of E-cadherin may be a reliable indicator of increase in invasiveness in oral squamous cell carcinoma. Das RK et al., observed E-cadherin expression in Oral Submucous Fibrosis (OSF) and concluded that expression of E-cadherin, a marker for cell to cell adhesiveness, was reduced in all OSF conditions, the reduction was greatest in OSF showing severe epithelial dysplasia [27].
Further in malignant group, the grade of expression of was strong (56%) but less as compared to premalignant lesions (87.5%, p=0.006). Within the malignant group well differentiated SCC showed a strong pattern (++) of immunostaining in 92.3% cases. While, moderately and poorly differentiated tumours showed heterogeneous staining (+/0) in 41.6% (majority) of cases, where both positive and negative areas of immunostaining were found and eventually lost in some cases (8.3%). This loss of E-cadherin can be explained by theory of mesenchymal transition by squamous cells on poor differentiation or loosening of cell to cell adhesion and getting more invasive property [8,23]. Schipper JH et al., studied E-cadherin in 32 cases of SCC of head and neck region. They observed that E-cadherin expression is inversely correlated both with the loss of epithelial morphology and tumour metastasis. The well-differentiated SCCs strongly expressed E-cadherin, similarly as the normal stratified epithelium while the moderately differentiated SCCs expressed heterogeneous pattern of E-cadherin and poorly differentiated SCCs lost E-cadherin expression [28]. Kaur G et al., in their study concluded that E-cadherin plays an important role in progression of Oral SCC, i.e. down regulation of its expression is associated with poor differentiation and metastasis [21].
On further analysis of qualitative expression of E-cadherin with degree of differentiation of SCC’s revealed that as we progress from well to poor differentiation; there is gradual loss of E-cadherin staining; from strong membranous to become faint cytoplasmic. The 50% cases of moderately/poorly differentiated tumour showed only cytoplasmic staining (score-4 pattern) in contrast to only 15.4% well differentiated tumour. Many authors have tried to explain this shift of E-cadherin as follows. After loss of intercellular contact results in the endocytic uptake of desmosomal glycoprotein in membrane vesicles. It remains associated with the plaque component and is seen as clumps. Breakdown of these membranous vesicles results in a diffuse cytoplasmic staining of E-cadherin [7,21,29]. Kaur G et al., [21] also found a decrease in membranous staining and shift towards cytoplasmic staining as the tumour become more differentiated. Our finding is supported by study of Akhtar K et al., and Balasundaram P et al., [30,31]. They concluded that invasiveness and recurrence could be analysed by the IHC staining pattern of E-cadherin and vimentin, which can help in predicting the tumour behaviour, prognosis, survival and management of the patient. Similarly, Rodriguez-sastrea MA et al., compared the distribution of E-cadherin in normal ectocervical epithelium, pre-malignant lesion and SCC’s and observed that E-cadherin exhibited a significant abnormal distribution in cytoplasm of SCC’s and premalignant lesions as compared to normal tissue. These finding suggested that cellular changes of E-cadherin were frequent in tumours of cervical cancer of different histologic types which supported the role of E-cadherin in cervical cancer development [32].
In this study, on comparison of qualitative assessment of E-cadherin expression among cervical and oral samples statistically insignificant difference was observed suggesting that E-cadherin is equally efficacious marker for both oral and cervical lesions.
Limitation
Limitations of this study are small sample size, variation in the expression of E-cadherin marker, different cofactors in carcinogenesis of oral and cervical cancers. In future E-cadherin should be studied with mesenchymal markers on larger sample to establish the role of E-cadherin in squamous cell lesions.
Conclusion
The qualitative and quantitative immunostaining characters of E-cadherin are quite characteristic and equally efficacious in oral and cervical premalignant and malignant lesions. It can be used as a significant biomarker for diagnostic and prognostic purpose in oral and cervical premalignant and malignant lesions.
χ2=0.216 (df=2), p=0.898
χ2=10.330 (df=2), p=0.006 (Kruskal-Wallis test)
(0 = Absence, +/0 = Heterogeneous, + = Weak, ++ = Strong)
z= 3.748, p<0.001
z= 1.505, p=0.205 (1= Absent staining, 2= Membranous staining, 3= Membranous and Cytoplasmic staining, 4= cytoplasmic staining)
χ2= 26.915 (df=2), p<0.001 (Kruskal-Wallis test)
[1]. Zhou J, Tao D, Xu Q, Gao Z, Tang D, Expression of e-cadherin and vimentin in oral squamous cell carcinomaInt J Clin Exp Pathol 2015 8(3):3150-54. [Google Scholar]
[2]. Mehendiratta M, Solomon MC, Boaz K, Guddattu V, Mohindra A, Clinico-pathological correlation of E-cadherin expression at the invasive tumor front of Indian oral squamous cell carcinomas: An immunohistochemical studyJ Oral Maxillofac Pathol 2014 18(2):217-22.10.4103/0973-029X.14075325328302 [Google Scholar] [CrossRef] [PubMed]
[3]. Ali F, Kuelker R, Wassie B, Understanding cervical cancer in the context of developing countriesAnn Trop Med Public Health 2012 5(1):3-15.10.4103/1755-6783.92871 [Google Scholar] [CrossRef]
[4]. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A, Global cancer statistics, 2012CA Cancer J Clin 2015 65(2):87-108.10.3322/caac.2126225651787 [Google Scholar] [CrossRef] [PubMed]
[5]. Neville BW, Day TA, Oral cancer and precancerous lesionsCA cancer J Clin 2002 52(4):195-215.10.3322/canjclin.52.4.19512139232 [Google Scholar] [CrossRef] [PubMed]
[6]. Guilford P, E-cadherin downregulation in cancer: fuel on the fire?Mol Med Today 1999 5:172-177.10.1016/S1357-4310(99)01461-6 [Google Scholar] [CrossRef]
[7]. Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR, Cadherin switchingJ Cell Sci 2008 121(pt6):727-735.10.1242/jcs.00045518322269 [Google Scholar] [CrossRef] [PubMed]
[8]. Thiery JP, Acloque H, Huang RYJ, Nieto MA, Epithelial-mesenchymal transitions in development and diseaseCell 2009 139(5):871-90.10.1016/j.cell.2009.11.00719945376 [Google Scholar] [CrossRef] [PubMed]
[9]. Charalabopoulos A, Golias C, E-cadherin expression in Barrett’s esophagus and esophageal carcinomaEsophagus 2014 11(3):153-161.10.1007/s10388-014-0424-x [Google Scholar] [CrossRef]
[10]. Liu X, Chu K-M, E-Cadherin and Gastric Cancer: Cause, Consequence, and ApplicationsBioMed Research International 2014 Article ID 637308, 9 pages10.1155/2014/63730825184143 [Google Scholar] [CrossRef] [PubMed]
[11]. Tsanou E, Peschos D, Batistatou A, Charalabopoulos A, Charalabopoulos K, The E-cadherin adhesion molecule and colorectal cancerA global literature approaches. Anticancer Res 2008 28(6A):3815-26. [Google Scholar]
[12]. Singhai R, Patil VW, Jaiswal SR, Patil SD, Tayade MB, Patil AV, E-Cadherin as a diagnostic biomarker in breast cancerN Am J Med Sci 2011 3(5):227-233.10.4297/najms.2011.322722558599 [Google Scholar] [CrossRef] [PubMed]
[13]. Kase S, Sugio K, Yamazaki K, Okamoto T, Yano T, Sugimachi K, Expression of E-cadherin and beta-catenin in human non-small cell lung cancer and the clinical significanceClin Cancer Res 2000 6(12):4789-96. [Google Scholar]
[14]. Pignatelli M, Ansari TW, Gunter P, Liu D, Hirano S, Takeichi M, Loss of membranous E-cadherin expression in pancreatic cancer: correlation with lymph node metastasis, high grade, and advanced stageJ Pathol 1994 174(4):243-48.10.1002/path.17117404037884585 [Google Scholar] [CrossRef] [PubMed]
[15]. Xusheng Ren, Jianning Wang, Xuefen Lin, Xuxia Wang, E-cadherin expression and prognosis of head and neck squamous cell carcinoma: evidence from 19 published investigationsOnco Targets Ther 2016 9:2447-2453.10.2147/OTT.S9857727217768 [Google Scholar] [CrossRef] [PubMed]
[16]. Carico E, Atlante M, Bucci B, Nofroni I, Vecchione A, E-cadherin and alpha-catenin expression during tumor progression of cervical carcinomaGynecol Oncol 2001 80(2):156-61.10.1006/gyno.2000.603511161853 [Google Scholar] [CrossRef] [PubMed]
[17]. Kaplanis K, Kiziridou A, Liberis V, Destouni C, Galazios G, E-cadherin expression during progression of squamous intraepithelial lesions in the uterine cervixEur J Gynaecol Oncol 2005 26(6):608-10. [Google Scholar]
[18]. Sridevi U, Jain A, Nagalaxmi V, Kumar UV, Goyal S, Expression of E-cadherin in normal oral mucosa, in oral precancerous lesions and in oral carcinomasEur J Dent 2015 9(3):364-72.10.4103/1305-7456.16323826430364 [Google Scholar] [CrossRef] [PubMed]
[19]. Broders A, The microscopic grading of cancerSurg Clin North Am 1941 21:947-62. [Google Scholar]
[20]. Barnes L, Eveson JW, Reichart P, Sidransky D, World Health Organization Classification of TumoursPathology and Genetics of Head and Neck Tumours 2005 LyonIARC Press [Google Scholar]
[21]. Kaur G, Carnelio S, Rao N, Rao L, Expression of E-cadherin in primary oral squamous cell carcinoma and metastatic lymph nodes: an immunohistochemical studyIndian J Dent Res 2009 20(1):71-76.10.4103/0970-9290.4907519336864 [Google Scholar] [CrossRef] [PubMed]
[22]. Wijnhoven BP, Dinjens WN, Pignatelli M, E-cadherin-catenin cell-cell adhesion complex and human cancerBr J Surg 2000 87(8):992-1005.10.1046/j.1365-2168.2000.01513.x10931041 [Google Scholar] [CrossRef] [PubMed]
[23]. Shinohara M, Hiraki A, Ikebe T, Nakamura S, Kurahara S, Shirasuna K, Immunohistochemical study of desmosomes in oral squamous cell carcinoma: Correlation with cytokeratin and E-cadherin staining, and with tumour behaviourJ Pathol 1998 184(4):369-81.10.1002/(SICI)1096-9896(199804)184:4<369::AID-PATH1236>3.0.CO;2-L [Google Scholar] [CrossRef]
[24]. Chen CL, Liu SS, Ip SM, Wong LC, Ng TY, Ngan HY, E-cadherin expression is silenced by DNA methylation in cervical cancer cell lines and tumoursEur J Cancer 2003 39(4):517-523.10.1016/S0959-8049(02)00175-2 [Google Scholar] [CrossRef]
[25]. Yogesh TL, Narayan T, Shreedhar B, Shashidara R, Leekymohanty The expression of E-cadherin and cathepsin-D in normal oral mucosa, oral epithelial dysplasia and oral squamous cell carcinoma: A comparative analysis between immunohistochemistry and routine histopathologyJ Oral Maxillofac Pathol 2011 15(3):288-94.10.4103/0973-029X.8668922144831 [Google Scholar] [CrossRef] [PubMed]
[26]. Monal B, Yuwanati J, V. Tupkari, Avadhoot Avadhani, Expression of E-cadherin in oral epithelial dysplasia and oral squamous cell carcinoma: An in vivo studyJ Clin Exp Invest 2011 2(4):347-353.10.5799/ahinjs.01.2011.04.0070 [Google Scholar] [CrossRef]
[27]. Das RK, Pal M, Barui A, Paul RR, Chakraborty C, Ray AK, Assessment of malignant potential of oral submucous fibrosis through evaluation of p63, E-cadherin and CD105 expressionJ Clinical Pathol 2010 63(10):894-899.10.1136/jcp.2010.07896420876321 [Google Scholar] [CrossRef] [PubMed]
[28]. Schipper JH, Frixen UH, Behrens J, Unger A, Jahnke K, Birchmeier W, E-Cadherin expression in squamous cell carcinomas of head and neck: inverse correlation with tumor dedifferentiation and lymph node metastasisCancer Res 1991 51(23pt1):6328-6337. [Google Scholar]
[29]. Sorscher SM, Russack V, Cagle M, Feramisco JR, Green MR, Immunolocalization of E-cadherin in human head and neck cancerArch Pathol Lab Med 1995 119(1):82-84. [Google Scholar]
[30]. Akhtar K, Anjum Ara A, Siddiqui SA, Sherwani RK, Diagnostic and Prognostic Significance of E-Cadherin and Vimentin in Oral Cancer MetastasisAnn Pathol Lab Med 2016 3(1):9-13. [Google Scholar]
[31]. Balasundaram P, Singh M.K., Dinda A.K., Thakar A, Yadav R, Study of β-catenin, E-cadherin and vimentin in oral squamous cell carcinoma with and without lymph node metastasesDiagn. Pathol 2014 9:14510.1186/1746-1596-9-14525047112 [Google Scholar] [CrossRef] [PubMed]
[32]. Rodrıguez-Sastrea MA, Gonzalez-Maya L, Delgado R, Lizano M, Tsubaki G, Mohar A, Abnormal distribution of E-cadherin and h-catenin in different histologic types of cancer of the uterine cervixGynecologic Oncology 2005 97(2):330-336.10.1016/j.ygyno.2004.12.06215863126 [Google Scholar] [CrossRef] [PubMed]