Introduction
Most developed countries including UK and North America are mostly prone to be affected with the periodontal disease [1,2]. Periodontal disease is basically classified into two types: (1) Gingivitis; (2) Periodontitis [3]. Gingivitis meant for the infection and inflammation of gingiva without any irreversible damage to the underlying deeper periodontal structure. In the earliest stage, the infection affects only the gingiva, which later spread to the deeper parts of supporting tissues. In periodontitis the detachment of periodontal ligament from the cementum occur, which subsequently leads to apical migration of junctional epithelium, pocket formation, gingival recession and alveolar bone destruction that finally results into tooth loss, if not treated properly [4].
The virulence of periodontal pathogens is determined by the type of toxins released by them and the type of inflammatory substances gets activated in response to it. Periodontal disease is basically a chronic infectious disease that has been linked to various systemic inflammatory conditions. In different organ systems, migration of periodontal pathogens like Porphyromonas gingivalis from mouth to brain contributes for low but persistent level of local inflammation. Liberation of reactive oxygen/nitrogen species also takes place when innate immune responses are activated by Aβ [5] from cell walls of bacteria [6]. The pathogen further increases the concentration of proinflammatory cytokines like Tumour Necrosis Factor α, (TNF-α) reduces the anti-inflammatory mediators like interleukin 1 [7] and also increases the concentration of C Reactive Protein (CRP) [8]. An elevated concentration of cytokines are resulted in patients of Alzheimer’s Disease (AD) which is because of acute and chronic inflammatory responses in body [4,7]. Studies revealed that there is higher incidence of periodontal disease (periodontitis) in patients with neurodegenerative disorders [9,10] and there is an increase in the expression of higher amyloid precursor proteins in periodontitis [11,12] and higher deposition of plaques of Aβ in the elderly population [13]. Thus, periodontal infection leads to survivability of harmful microbes, loss of beneficial microbes in the oral cavity ultimately leading to the decreased immunity of host and oral health related quality of life in an elderly population [14].
Aetiology and pathogenesis of neurodegenerative diseases are not well characterised; however, inflammation is thought to play a remarkable role. Studies have revealed that peripheral infections e.g. periodontal diseases can accelerate the onset and progression of neurodegenerative diseases; although, mechanisms of pathway through which it affects, are not well understood [2,6,12-15]. The objective of this review is to present possible pathway and contributions between periodontal diseases and pathogenesis of neurodegeneration and also to discuss the role of free radicals in prevention and treatment of periodontal disease induced neurodegeneration.
Microflora Altered during Mouth Infection
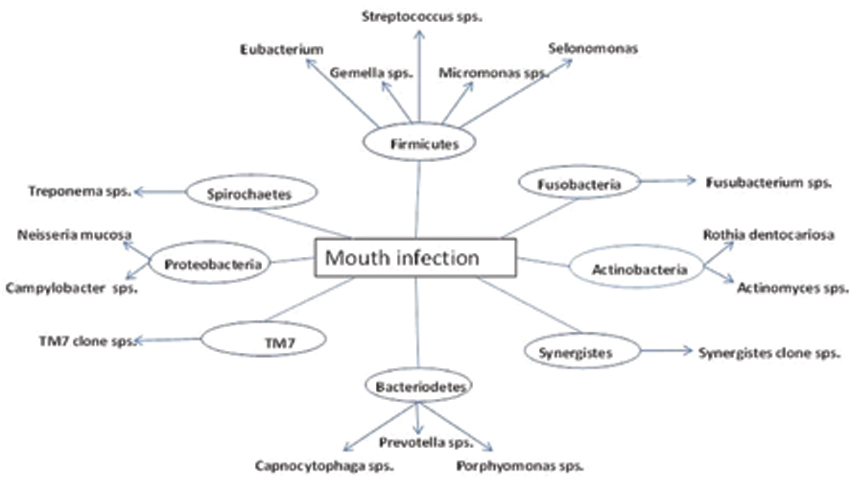
Various microbes are found in the oral cavity of a healthy human being. The plethora of microflora residing as commensal in human being is appraised to have outskirt the total number of cells in the body [16]. It is the inherent property of oral cavity which provides the opportunity for miscellaneous growth of microbiota. The microbes have two habitat preferences for them, one being the shedding (mucosa) and other being the solid surfaces (teeth or dentures) [17]. The microbiota includes Bacteroidetes, Firmicutes, Tenericutes, Actinobacteria, Proteobacteria, Euryarchaeota, Chlamydiae, Spirochaetes, Streptococci, Actinomycetes, Veillonella, and Diphtheroids, and gram negative anaerobic rods [17]. In humans the oral cavity found to contain over 700 species [18]. Out of 700 species, the periodontal pocket contains more than 400 species, and the rest 300 species are found to be present at other oral sites like mucosal membranes, lesions, tongue, and endodontic infections, which was established on the analysis of 16S rRNA clones [18]. The eight different phyla contribute for oral infection, which includes Firmicutes, Synergistes, Spirochaetes, Actinobacteria, Proteobacteria, Bacteroidetes, Fusobacteria and TM7 phylum [Table/Fig-1] [19].
Various microbial family contributing for oral infection.
Immune Invasion
Bacteria present in the oral cavity invade host immune barrier by destroying the complement fragments. A complex of complement proteins includes C1-C9, which together form Membrane Attack Complex (MAC). Members of this family have the ability to create pore across the membrane. Bacterial infection degrade C3 and C5 protein by a trypsin-like cysteine proteinases named gingipains [20], which avoids the opsonisation mechanism mediated by the C3b by attaching on the surface of the bacterial cell walls. Mostly the gingipains are found to be present on the surface of the bacterial cell wall and degrade complement proteins of host [21]. Gingipains prevent its degradation by complement mediated lysis by attaching to C4b binding protein [21]. Gingipains are major cause in the development of periodontitis, by mediating swelling and destroying the tissue in the periodontium of teeth [22]. P. gingivalis infection leads to two types of gingipains. One is specific for lysine binding (Kgp) and another is specific for arginine (Rgps) depending upon the specificity for their cleaving sites [20].
The main key for host pathogen interaction during infection is the recognition of Lipopolysaccharides (LPS). LPS are the main signals present over cell wall of pathogens and this recognition is mediated via a receptor of host cell surface that is toll-like receptor, after which the enzymes as well as toxins of microbes, get access to the blood stream of host through the ulcerated epithelium of the periodontal pocket. From there gram-negative bacteria enter to the bloodstream by releasing toxins and inducing immune response by releasing proinflammatory mediators such as TNF-α, prostaglandin E2, and interleukin-1β, γ-interferon [23]. The elevated levels of infectious agents which decrease the self-tolerance capacity of host organism leading to the onset of autoimmune disease [24].
Poor oral hygiene and periodontal pathogens seem to influence the incidence of various systemic disorders [4] e.g., the receptors in the oral mucosal surface encourage attachment and colonisation of respiratory pathogens which subsequently leads to lower respiratory tract and lung infection [25]. Studies have also found that there is an increase in inflammatory serum markers such as IL-6 fibrinogen and CRP in patients having periodontitis than patients without periodontitis [26]. In the same way, chronic periodontitis and disorders of metabolism are the key to systemic inflammation and insulin resistance [27].
Periodontitis proceeds to neurodegenerative diseases by microbial or inflammatory infection (2). In the first mechanism, by neuronal pathways, T. denticola multiplication affects branches of the trigeminal nerve [2,28]. In the second method by release of inflammatory molecules like CRP, IL-6, IL-1β, IL8 and TNF-α inflammation increases in brain [28].
Microbes and Neurodegeneration in various Diseases
During metabolism microbes interact with O2 and metal ions to produce Reactive Oxygen Species (ROS) like peroxides, hydroxyl ions, superoxide anions [29]. ROS are produced by two pathways [30].
(1) Direct interactions between REDOX-active metals and oxygen species;
(2) Indirect pathway drives through metalloenzymes like xanthine dehydrogenase, nitric oxide synthase and phospholipases which are the activators of calcium.
ROS are very reactive in nature and initiate cell death in neurons as well as mediate mutations in DNA by interacting with it. Oxidative stress creates cellular dysfunctions resulting information of toxic species like cholesterol oxides, peroxides, aldehydes, ketones and alcohols. Cholesterol oxide has a toxic effect on lymphocytes and macrophages residing on blood vessels [31]. ROS, a redox signal, generated because of the enzymatic reactions, and thus acts as a second messenger mediating a number of signalling systems [32]. Excessive ROS impairs intracellular calcium signalling leading to different apoptotic cascade, tissue injury, inflammation, brain injury and degenerative diseases [33]. Effect of ROS in various neurodegenerative diseases are explained below.
Alzheimer’s disease (AD): Mutation in the amyloid precursor protein leads to deposition of small fragments of Aβ peptide in brain of AD patients [34]. Specifically ROS generated by pathogenicity of periodontal bacteria cause oxidative modification which generate abnormal soluble forms of Aβ from the membrane [35].
A variety of systemic changes were found in an experimental mouse model (ApoE null mouse) that was infected by P. gingivalis and T. denticola. In that study the brains of mice were found to contain the genomic DNA of P. Gingivalis and again chronic gingival was analysed by nucleotide sequencing method [36].
Parkinson’s disease: In Parkinson’s Disease (PD), there is accumulation of α-synuclein. Due to a missense mutation it leads to the destruction of dopaminergic neurons in the substantia nigra and the accumulation of intracellular inclusion bodies. The mutations in α-synuclein, which are parts of the deposits, is mainly mediated through the ROS generation during microbial infection in case of the oral periodontitis, which affects the expression of one enzyme dihydropteridinereductase, that maintains the production of dopamine [37]. With the onset of aging a dark brown pigment called, neuromelanin gets accumulated in substantia nigra of brain that accumulates metal ions, basically iron, which is an inflammatory agent. In Parkinson’s disease, these metal accumulating agents are lost [38].
ApoE null mouse model is used to study Parkinson’s disease. Studies to describe the strong intracellular labelling with C3 and C9, researchers use antibodies against C3 convertase and the membrane attack complex of brain tissue sections from the ApoE null mice. Microglia in both infected and uninfected groups, demonstrated solves the query [39]. Glia cells are more subjected to attack by MAC complex [40]. Opsonisation with complement activation fragments iC3b/C3b/C3d has also been found after local intravascular dissemination of P. gingivalis into brain tissue [41].
Relationship between Neurodegeneration and Periodontal Infection
Inflammation has principal role in the connection between periodontitis and neurodegenerative diseases. Inflammation is a protective mechanism that defends the body from external and internal agent or stimuli. In central nervous system, the main innate immune-defensive cells are resident microglial cell [42]. In systemic inflammation, inflammatory mediators are released that help in microglial cells activation [43], which leads to initiation of neurodegeneration. It suggests that periodontitis can direct the progression of neurodegenerative diseases similar to Alzheimer’s disease, Huntington disease, Parkinson’s disease by two plausible mechanisms [2].
a) Periodontitis leads to systemic inflammation/infection;
b) Bacterial and viral influence.
Both the mechanisms follow these three pathways [44], that are: (1) pathogen invasion and liberate of inflammatory mediators beginning from the oral cavity into the systemic circulation; (2) systemic inflammation occurred by periodontitis can affect the central nervous system through various possible ways; (3) the declination of dopaminergic neurons as well as activation of microglia occur in the substantia nigra which is located in midbrain [Table/Fig-2].
Infection mode of pathogen from oral cavity to brain.
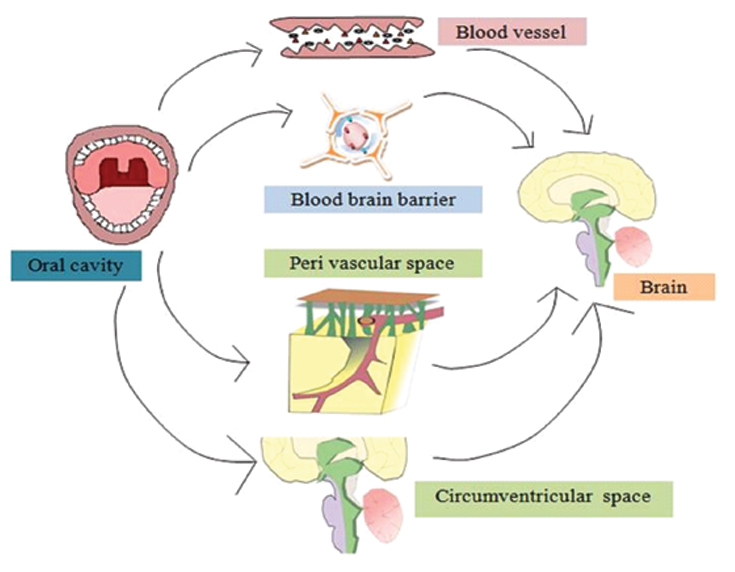
According to first pathway, under normal condition, the oral cavity acts as a physical, chemical, immunological barrier and prevents the entry of the microorganism. To increase the acute and chronic infection microbes have to acquire host system, for which it has to overcome all the barriers and then evade and spread infection [45]. Some specific bacteria form dental plaque or biofilm by stimulating proinflammatory cytokines, including IL-1β, IL-6 and TNF-α [45]. The proinflammatory mediator TNF-α expression increases in Alzheimer’s disease and it is known to be the pivotal inflammatory cytokine. It facilitates the flow of events occurred in neuroinflammatory response. TNF-α exacerbates demyelination, inflammation, gliosis, cell death and Blood Brain Barrier (BBB) deterioration [7,45]. So TNF-α plays crucial role in the neurodegenerative disease. In advanced stages, periodontal disease may produce systemic inflammation. According to second pathway, an inflammatory response created both locally as well as systemically due to periodontal infection. From periphery the proinflammatory molecules are secreted help in boosting total pool of brain inflammatory molecules which are passed through systemic circulation and neural pathway or humoral pathway [3]. In the systemic circulation, proinflammatory molecules might enter the CNS through multiple pathways. They may enter the CNS through the areas of the brain which have no BBB (blood brain barrier) (circumventricular organ) [Table/Fig-3]. Also, sometimes through BBB, these molecules can enter the CNS by: (1) passing across fenestrated capillaries of BBB; (2) through transporters which is cytokine-specific; (3) BBB permeability increases; (4) brain endothelial cells activate and generate signalling molecules which associate with cytokine such as nitric oxide or prostanoids [3]. In humoral pathway, BBB is included. BBB allow any substance to pass from blood to brain. Due to infection the chances of disruption of BBB is high, which can lead to release of proinflammatory cytokines [46]. ROS and nitric oxide produced by activated microglial cell leads to dopaminergic neuronal death [3,46].
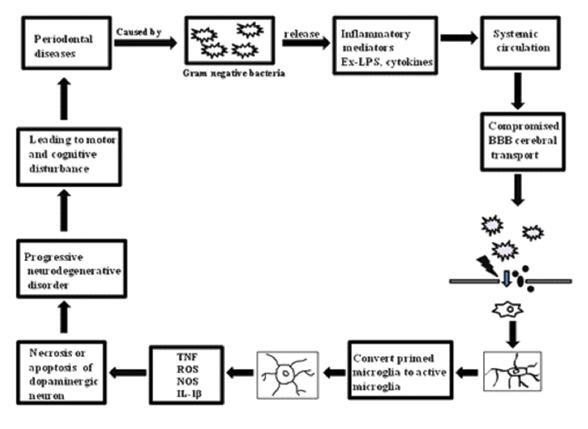
Proposed pathway between neurodegenerative disease and periodontal disease.
The principal cause of periodontitis is dental plaque which exists in biofilm form. The anaerobic species and gram negative bacteria colonise or invade the intact epithelium. Periodontal pocket give large surface area and ulceration in pocket lining help the pathogen to enter into systemic circulation [47]. In periodontitis, primarily some pathogens are involved. These are Treponema denticola (gram-negative, obligate anaerobic, pathogenic), Porphyromonas gingivalis (gram-negative, anaerobic, pathogenic), Eikenella corrodens (gram-negative, facultative anaerobic, pathogenic), Fusobacterium nucleatum (gram-negative, anaerobic, pathogenic), Tannerellaforsythia (gram-negative, anaerobic, pathogenic), Prevotellaintermedia (gram-negative, obligate anaerobic, pathogenic), and Aggregatibacteractinomycetemcomitans (gram-negative, facultative anaerobic, pathogenic) [48,49]. Specifically P. gingivalis, A actinomycetemcomitans, and T. denticola help in tissue invasion. Periodontal pathogens with their products are able to enter to the circulation predominantly in the ulcerated pockets, and subsequently in systemic circulation.
Bacterial associated products with proinflammatory molecules can enhance the brain proinflammatory cytokine pool. The gram negative bacterial LPS, readily recognise molecule to induce innate immune responses and CD14 receptor activate the peripheral proinflammatory cytokines. Several studies have shown that CD14 receptor found on specific area of the brain such as circumventricular areas, leptomeninges, and choroid plexus are exposed to systemic circulation [21,50]. LPS injected in intravenously and it up regulates CD14 receptors throughout the brain not only the specific areas which exposed to the LPS [50]. The breakdown of the BBB and the granulocytes or soluble molecules invasion might be caused due to systemic injection of LPS [51]. A reduced number of dopaminergic neurons resulted because of intranigral injection of LPS specially at substantia nigra [52]. Several studies shown that LPS-induced neurotoxicity is seen only in dopaminergic neuron not in GABAergic or the serotonergic neuron [52,53]. Altogether, the LPS is an essential cause for the development and progression of Parkinson’s disease.
According to third pathway, the LPS of the gram-negative bacteria breached the BBB and provide the way of entrance for the macrophages and proinflammatory mediators, which developed active microglia from primed microglia. After activation of primed microglia, it produces several inflammatory mediators such as ROS, IL-6, TNF-α, IL-1β and CRP. Elevated CRP and proinflammatory cytokines might stimulate glial cells via paracrine and/or autocrine pathways to produce additional P-Tau, Aβ42 and proinflammatory molecules. Thus, a cycle is established in which inflammatory mediators play a dual role by both accelerating glial cells and triggering molecular pathways, might lead to necrosis and lastly dopaminergic neuron apoptosis. The dopaminergic neuron death marks the initiation and progression of neurodegenerative disease [2,3] [Table/Fig-3].
Antioxidants in the Prevention and Treatment of Neurodegenerative Disorders
Various antioxidants are used to prevent neurodegeneration. Polyphenols (PPs) have a unique property which is beneficial for health. PPs prevent degenerative diseases, any infectious diseases and also oral diseases via many mechanisms [54]. PPs are plant metabolites which are present in the form of fruits, seeds and leaves. They possess several phenol groups (i.e., aromatic rings with hydroxyls), which are derived from L-phenylalanine [54-56]. Polyphenols exert antibacterial and anti-oxidative property [57]. Usually free radicals are neutralised by antioxidants like polyphenols so that it can diminish their destructive effects [58]. Most age related degenerative diseases caused due to oxidative damage, have protective role via PP [59].
Polyphenol
Periodontal pathogens stimulate inflammation by which it increases the crevicular fluid production and chemotaxis of polymorphonuclear leukocytes, which inactivate the periodontal pathogen and release hypochlorous acid and singlet oxygen into the crevicular fluid [54]. Antioxidant activity of ascorbate, albumin and urate enhance the oxidative stress which is present in the crevicular fluid derived from plasma. Exterior factor or some systemic condition such as smoking, diabetics and metabolic syndrome induced local oxidative stress. Disequilibrium between oxidative stress and antioxidant activity leads to destruction of periodontal tissues [54]. Polyphenol helps to enhance the oral fluids antioxidant activity [59,58]. These observations suggest that antioxidant rich diet help in inhibition of periodontal development and progression, predominantly in subjects exposed to dietary and environmental sources of oxidative stress [60-63]. Studies further reveal the relation between the crevicular fluid and saliva antioxidative activity with periodontitis development. When antioxidant activities of crevicular fluid and saliva decrease, leading to the development of periodontal disease [64-66]. The PPs present in green or black tea which reside in the mouth for 2-5 minutes, it increases the saliva anti-oxidant capacity [67]. A daily dose of two grapes for up to two weeks can act as a great source in enhancing phagocytic activity of polymorphonuclear leucocytes [68]. PPs possess antibacterial activity against pathogens associated to periodontitis and help in prevention of biofilm formation even decrease pocket depth with local application. Several studies reveal that the green tea PPs inhibitory effect on the virulence factor which are produced from the periodontal disease causing anaerobic bacterium Porphyromonas gingivalis [69]. Daily consumption of tea PPs can be useful and practical method for the prevention of periodontal diseases.
Vitamin E (α-tocopherol)
Vitamin E is an antioxidant that breaks the extension of the free radical chain reaction in lipid regions of membrane. Studies revealed that it can be a potent drug for curing Alzheimer’s disease [70]. Vitamin E is fat soluble in nature and comes into systemic circulation after getting absorbed from small intestine [71]. Vitamin E concentration in plasma level is maintained by liver through the absorption of LDL to the plasma. Transportation of LDL is through a transfer protein by α tocopherol [72]. If any genetic problem may appears in this protein, it may leads to vitamin E deficiency in human which induce peripheral neuropathy [73].
Vitamin C (Ascorbic Acid)
Vitamin C cannot be synthesised by human and other primates, while most mammals generate it endogenously in the liver [74]. Dehydroascorbate, which is the oxidised form of vitamin C, hydrolysis to form 2,3-diketo-L-gulonic acid and mediate release of carbon dioxide and components of pentose phosphate cycle. The synthesis of vitamin E can be mediated through renewal of vitamin C [75]. Vitamin C concentration is found to be high in brain. It is a cofactor of dopamine β-hydroxylase, and biosynthesis of catecholamine. It also acts as a free radical scavenger [76]. Vitamin C enters the brain and retain as ascorbic acid. The oxidised form of ascorbic acid is able to enter the BBB through GLUT1 receptor. The investigators concluded that, the blood concentrations of dehydroascorbic acid directly proportional to the vitamin C concentration in brain [12].
Recent literature has consistently shown an association between periodontal diseases and neurodegeneration [10-12]. So, the possibility that periodontal infections may contribute to neurodegenerative diseases pathogenesis can’t be excluded. Hence, more number of interventional studies and multicentre longitudinal studies, with large sample sizes should be carried out to prove causal relationship between periodontal infections and neurodegeneration. Also, preventive measures like use of antioxidants and supportive periodontal therapy should be taken to guarantee neurodegenerative patients a high quality of life.
Conclusion
Neurodegenerative disorders involve a complex pathophysiology and exact etiopathogenesis is still unknown. Presence of chronic inflammation has been suggested as a central mechanism for neurodegenerative disorders which is the common link between periodontal diseases and neurodegeneration. Like periodontitis, ROS is the key mediator in the progression of neurodegenerative disease which is released due to bacterial invasion into host cells. Possible pathway and contributions from periodontal infections and role of antioxidants in neurodegeneration have been discussed here. Presently, it may be stated that periodontal infections may pose as a potential risk factor for the development of neurodegenerative disorders. Patients, particularly of older age group should be strongly motivated towards oral hygiene maintenance and periodontal care, in mutual coordination of dental professionals and neurologist.
[1]. Albandar JM, Rams TE, Global epidemiology of periodontal diseases: an overviewPeriodontol 2000 2002 29(1):07-10.10.1034/j.1600-0757.2002.290101.x [Google Scholar] [CrossRef]
[2]. Gurav AN, Alzheimer’s disease and periodontitis-an elusive linkRevista da Associação Médica Brasileira 2014 60(2):173-80.10.1590/1806-9282.60.02.01524919005 [Google Scholar] [CrossRef] [PubMed]
[3]. Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik-Sobanska L, de Leon MJ, Inflammation and Alzheimer’s disease: possible role of periodontal diseasesAlzheimer’s Dement 2008 4(4):242-50.10.1016/j.jalz.2007.08.00418631974 [Google Scholar] [CrossRef] [PubMed]
[4]. Pizzo G, Guiglia R, Russo LL, Campisi G, Dentistry and internal medicine: from the focal infection theory to the periodontal medicine conceptEur J Intern Med 2010 21(6):496-502.10.1016/j.ejim.2010.07.01121111933 [Google Scholar] [CrossRef] [PubMed]
[5]. Rosales-Corral SA, Acuña-Castroviejo D, Coto-Montes A, Boga JA, Manchester LC, Fuentes-Broto L, Alzheimer’s disease: pathological mechanisms and the beneficial role of melatoninJ Pineal Res 2012 52(2):167-202.10.1111/j.1600-079X.2011.00937.x22107053 [Google Scholar] [CrossRef] [PubMed]
[6]. Rangarajan M, Aduse-Opoku J, Paramonov N, Hashim A, Bostanci N, Fraser OP, Identification of a second lipopolysaccharide in Porphyromonas gingivalis W50J Bacteriol 2008 190(8):2920-32.10.1128/JB.01868-0718263730 [Google Scholar] [CrossRef] [PubMed]
[7]. Passoja A, Puijola I, Knuuttila M, Niemelä O, Karttunen R, Raunio T, Serum levels of interleukin 10 and tumour necrosis factor α in chronic periodontitisJ Clin Periodontol 2010 37(10):881-87.10.1111/j.1600-051X.2010.01602.x20718895 [Google Scholar] [CrossRef] [PubMed]
[8]. Paraskevas S, Huizinga JD, Loos BG, A systematic review and meta analyses on C reactive protein in relation to periodontitisJ Clin Periodontol 2008 35(4):277-90.10.1111/j.1600-051X.2007.01173.x18294231 [Google Scholar] [CrossRef] [PubMed]
[9]. Frota BMD, Holanda SN, Sousa FB, Alves APNN, Evaluation of oral conditions in patients with neurodegenerative diseases treated in geriatric centersRev Gaúcha Odontol 2016 64(1):17-23.10.1590/1981-863720160001000022854 [Google Scholar] [CrossRef]
[10]. Cicciu M, Risitano G, Lo Giudice G, Bramanti E, Periodontal health and caries prevalence evaluation in patients affected by Parkinson’s diseaseParkinson’s Dis 2012 201210.1155/2012/54190823320249 [Google Scholar] [CrossRef] [PubMed]
[11]. Abbayya K, Puthanakar NY, Naduwinmani S, Chidambar Y, Association between periodontitis and Alzheimer’s diseaseN Am J Med Sci 2015 7(6):24110.4103/1947-2714.15932526199919 [Google Scholar] [CrossRef] [PubMed]
[12]. Hatipoglu MG, Kabay SC, Güven G, The clinical evaluation of the oral status in Alzheimer type dementia patientsGerodontology 2011 28(4):302-06.10.1111/j.1741-2358.2010.00401.x21054507 [Google Scholar] [CrossRef] [PubMed]
[13]. Kamer AR, Pirraglia E, Tsui W, Rusinek H, Vallabhajosula S, Mosconi L, Periodontal disease associates with higher brain amyloid load in normal elderlyNeurobiol Aging 2015 36(2):627-33.10.1016/j.neurobiolaging.2014.10.03825491073 [Google Scholar] [CrossRef] [PubMed]
[14]. Cicciù M, Matacena G, Signorino F, Brugaletta A, Cicciù A, Bramanti E, Relationship between oral health and its impact on the quality life of Alzheimer’s disease patients: a supportive care trialInt J Clin Exp Med 2013 6(9):766 [Google Scholar]
[15]. Cicciù M, Neurodegenerative disorders and periodontal disease: is there a logical connection?Neuroepidemiology 2016 47(2):94-95.10.1159/00044951727618223 [Google Scholar] [CrossRef] [PubMed]
[16]. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, A core gut microbiome in obese and lean twinsNature 2009 457(7228):480-84.10.1038/nature0754019043404 [Google Scholar] [CrossRef] [PubMed]
[17]. Scully C, Greenman J, Halitosis (breath odor)Periodontol 2000 2008 48(1):66-75.10.1111/j.1600-0757.2008.00266.x18715357 [Google Scholar] [CrossRef] [CrossRef]
[18]. Paster BJ, Olsen I, Aas JA, Dewhirst FE, The breadth of bacterial diversity in the human periodontal pocket and other oral sitesPeriodontol 2000 2006 42(1):80-87.10.1111/j.1600-0757.2006.00174.x16930307 [Google Scholar] [CrossRef] [PubMed]
[19]. da Silva APB, Bacterial Characterization in Health and Periodontal Diseases During Induced Gingival Inflammation 2012 University of North Carolina at Chapel Hill [Google Scholar]
[20]. Imamura T, The role of gingipains in the pathogenesis of periodontal diseaseJ Periodontol 2003 74(1):111-18.10.1902/jop.2003.74.1.11112593605 [Google Scholar] [CrossRef] [PubMed]
[21]. Popadiak K, Potempa J, Riesbeck K, Blom AM, Biphasic effect of gingipains from Porphyromonas gingivalis on the human complement systemJ Immunol 2007 178(11):7242-50.10.4049/jimmunol.178.11.724217513773 [Google Scholar] [CrossRef] [PubMed]
[22]. Miyachi K, Ishihara K, Kimizuka R, Okuda K, Arg-gingipain A DNA vaccine prevents alveolar bone loss in miceJ Dent Res 2007 86(5):446-50.10.1177/15440591070860051117452566 [Google Scholar] [CrossRef] [PubMed]
[23]. Hajishengallis G, Periodontitis: from microbial immune subversion to systemic inflammationNat Rev Immunol 2015 15(1):30-44.10.1038/nri378525534621 [Google Scholar] [CrossRef] [PubMed]
[24]. Liao F, Li Z, Wang Y, Shi B, Gong Z, Cheng X, Porphyromonas gingivalis may play an important role in the pathogenesis of periodontitis-associated rheumatoid arthritisMed Hypotheses 2009 72(6):732-35.10.1016/j.mehy.2008.12.04019246161 [Google Scholar] [CrossRef] [PubMed]
[25]. Paju S, Scannapieco F, Oral biofilms, periodontitis, and pulmonary infectionsOral Diseases 2007 13(6):508-12.10.1111/j.1601-0825.2007.01410a.x17944664 [Google Scholar] [CrossRef] [PubMed]
[26]. Mealey BL, Oates TW, Diabetes mellitus and periodontal diseasesJ Periodontol 2006 77(8):1289-303.10.1902/jop.2006.05045916881798 [Google Scholar] [CrossRef] [PubMed]
[27]. Lalla E, Papapanou PN, Diabetes mellitus and periodontitis: a tale of two common interrelated diseasesNat Rev Endocrinol 2011 7(12):738-48.10.1038/nrendo.2011.10621709707 [Google Scholar] [CrossRef] [PubMed]
[28]. Shoemark DK, Allen SJ, The microbiome and disease: reviewing the links between the oral microbiome, aging, and Alzheimer’s diseaseJ Alzheimer’s Dis 2015 43(3):725-38.10.3233/JAD-14117025125469 [Google Scholar] [CrossRef] [PubMed]
[29]. Mittler R, ROS are goodTrends Plant Sci 2017 22(1):11-19.10.1016/j.tplants.2016.08.00227666517 [Google Scholar] [CrossRef] [PubMed]
[30]. Cabiscol E, Tamarit J, Ros J, Oxidative stress in bacteria and protein damage by reactive oxygen speciesInt Microbiol 2010 3(1):03-08. [Google Scholar]
[31]. Chen X, Guo C, Kong J, Oxidative stress in neurodegenerative diseasesNeural Regen Res 2012 7(5):376 [Google Scholar]
[32]. Hsieh HJ, Liu CA, Huang B, Tseng AH, Wang DL, Shear-induced endothelial mechanotransduction: the interplay between Reactive Oxygen Species (ROS) and Nitric Oxide (NO) and the pathophysiological implicationsJ Biomed Sci 2014 21(1):310.1186/1423-0127-21-324410814 [Google Scholar] [CrossRef] [PubMed]
[33]. Radi E, Formichi P, Battisti C, Federico A, Apoptosis and oxidative stress in neurodegenerative diseasesJ Alzheimer’s Dis 2014 42(s3):S125-S52.10.3233/JAD-13273825056458 [Google Scholar] [CrossRef] [PubMed]
[34]. Murphy MP, LeVine III H, Alzheimer’s disease and the amyloid-β peptideJ Alzheimer’s Dis 2010 19(1):311-23.10.3233/JAD-2010-122120061647 [Google Scholar] [CrossRef] [PubMed]
[35]. Heneka MT, Kummer MP, Latz E, Innate immune activation in neurodegenerative diseaseNat Rev Immunol 2014 14(7):463-77.10.1038/nri370524962261 [Google Scholar] [CrossRef] [PubMed]
[36]. Singhrao SK, Harding A, Poole S, Kesavalu L, Crean S, Porphyromonas gingivalis periodontal infection and its putative links with Alzheimer’s diseaseMediators Inflamm 2015 2015:13735710.1155/2015/13735726063967 [Google Scholar] [CrossRef] [PubMed]
[37]. Gasser T, Genetics of Parkinson’s diseaseCurr Opin Neurol 2005 18(4):363-69.10.1097/01.wco.0000170951.08924.3d16003110 [Google Scholar] [CrossRef] [PubMed]
[38]. Nigam D, Free Radicals and Oxidative Stress in Neurodegenerative DisordersFree Radicals in Human Health and Disease 2015 Springer:143-58.10.1007/978-81-322-2035-0_11 [Google Scholar] [CrossRef]
[39]. Poole S, Aetiological links between oral pathogens and dementia 2014 University of Central Lancashire [Google Scholar]
[40]. Wyss-Coray T, Rogers J, Inflammation in Alzheimer disease-a brief review of the basic science and clinical literatureCold Spring Harb Perspect Med 2012 2(1):a00634610.1101/cshperspect.a00634622315714 [Google Scholar] [CrossRef] [PubMed]
[41]. Singhrao SK, Chukkapalli S, Poole S, Velsko I, Crean SJ, Kesavalu L, Chronic Porphyromonas gingivalis infection accelerates the occurrence of age-related granules in ApoE–/–mice brainsJ Oral Microbiol 2017 9(1):127060210.1080/20002297.2016.127060228326151 [Google Scholar] [CrossRef] [PubMed]
[42]. Ferrari CC, Tarelli R, Parkinson’s disease and systemic inflammationParkinson’s Dis 2011 2011:43681310.4061/2011/43681321403862 [Google Scholar] [CrossRef] [PubMed]
[43]. Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH, Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegenerationJ Neurosci 2005 25(40):9275-84.10.1523/JNEUROSCI.2614-05.200516207887 [Google Scholar] [CrossRef] [PubMed]
[44]. Kaur T, Uppoor A, Naik D, Parkinson’s disease and periodontitis-the missing link? a reviewGerodontology 2016 33(4):434-38.10.1111/ger.1218825664991 [Google Scholar] [CrossRef] [PubMed]
[45]. Cerajewska T, Davies M, West N, Periodontitis: a potential risk factor for Alzheimer’s diseaseBri Dent J 2015 218(1):29-34.10.1038/sj.bdj.2014.113725571822 [Google Scholar] [CrossRef] [PubMed]
[46]. Herrera A, Tomas-Camardiel M, Venero J, Cano J, Machado A, Inflammatory process as a determinant factor for the degeneration of substantia nigra dopaminergic neuronsJ Neural Transm 2005 112(1):111-19.10.1007/s00702-004-0121-315599609 [Google Scholar] [CrossRef] [PubMed]
[47]. Ebersole JL, Cappelli D, Acute phase reactants in infections and inflammatory diseasesPeriodontol 2000 2000 23(1):19-49.10.1034/j.1600-0757.2000.2230103.x [Google Scholar] [CrossRef]
[48]. Socransky SS, Haffajee AD, Periodontal microbial ecologyPeriodontol 2000 2005 38(1):135-87.10.1111/j.1600-0757.2005.00107.x15853940 [Google Scholar] [CrossRef] [PubMed]
[49]. Filoche S, Wong L, Sissons C, Oral biofilms: emerging concepts in microbial ecologyJ Dent Res 2010 89(1):08-18.10.1177/002203450935181219918089 [Google Scholar] [CrossRef] [PubMed]
[50]. Rivest S, Molecular insights on the cerebral innate immune systemBrain, Behavior, and Immunity 2003 17(1):13-19.10.1016/S0889-1591(02)00055-7 [Google Scholar] [CrossRef]
[51]. Bohatschek M, Werner A, Raivich G, Systemic LPS injection leads to granulocyte influx into normal and injured brain: effects of ICAM-1 deficiencyExp Neurol 2001 172(1):137-52.10.1006/exnr.2001.776411681847 [Google Scholar] [CrossRef] [PubMed]
[52]. Machado A, Herrera A, Venero J, Santiago M, De Pablos R, Villarán R, Inflammatory animal model for Parkinson’s disease: the intranigral injection of LPS induced the inflammatory process along with the selective degeneration of nigrostriatal dopaminergic neuronsISRN Neurol 2011 2011:47615810.5402/2011/47615822389821 [Google Scholar] [CrossRef] [PubMed]
[53]. Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B, Microglial activation mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s diseaseJ Neurochem 2002 81(6):1285-97.10.1046/j.1471-4159.2002.00928.x12068076 [Google Scholar] [CrossRef] [PubMed]
[54]. Petti S, Scully C, Polyphenols, oral health and disease: A reviewJ Dent 2009 37(6):413-23.10.1016/j.jdent.2009.02.00319303186 [Google Scholar] [CrossRef] [PubMed]
[55]. Knaggs AR, The biosynthesis of shikimate metabolitesNatural Product Reports 2003 20(1):119-36.10.1039/b100399m12636087 [Google Scholar] [CrossRef] [PubMed]
[56]. Boudet AM, Evolution and current status of research in phenolic compoundsPhytochemistry 2007 68(22):2722-35.10.1016/j.phytochem.2007.06.01217643453 [Google Scholar] [CrossRef] [PubMed]
[57]. Hannig C, Sorg J, Spitzmüller B, Hannig M, Al-Ahmad A, Polyphenolic beverages reduce initial bacterial adherence to enamel in situJ Dent 2009 37(7):560-66.10.1016/j.jdent.2009.03.01719394124 [Google Scholar] [CrossRef] [PubMed]
[58]. Ehrlich SD, Seed NG, University of Maryland medical centerGreen Tea [cited 2009 Nov 29]Available from: URL: http://www.umm.edu/altmed/articles/green-tea-000255 htm. 2013 [Google Scholar]
[59]. Weinreb O, Mandel S, Amit T, Youdim MB, Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseasesJ Nutr Biochem 2004 15(9):506-16.10.1016/j.jnutbio.2004.05.00215350981 [Google Scholar] [CrossRef] [PubMed]
[60]. Battino M, Bullon P, Wilson M, Newman H, Oxidative injury and inflammatory periodontal diseases: the challenge of anti-oxidants to free radicals and reactive oxygen speciesCrit Rev Oral Biol Med 1999 10(4):458-76.10.1177/1045441199010004030110634583 [Google Scholar] [CrossRef] [PubMed]
[61]. Sculley DV, Langley-Evans SC, Salivary antioxidants and periodontal disease statusProceedings of the Nutrition Society 2002 61(01):137-43.10.1079/PNS200114112002788 [Google Scholar] [CrossRef] [PubMed]
[62]. Ritchie CS, Kinane DF, Nutrition, inflammation, and periodontal diseaseNutrition 2003 19(5):47510.1016/S0899-9007(02)01043-2 [Google Scholar] [CrossRef]
[63]. Figuero E, Soory M, Cerero R, Bascones A, Oxidant/antioxidant interactions of nicotine, coenzyme Q10, pycnogenol and phytoestrogens in oral periosteal fibroblasts and MG63 osteoblastsSteroids 2006 71(13):1062-72.10.1016/j.steroids.2006.09.00317045317 [Google Scholar] [CrossRef] [PubMed]
[64]. Diab-Ladki R, Pellat B, Chahine R, Decrease in the total antioxidant activity of saliva in patients with periodontal diseasesClin Oral Investig 2003 7(2):103-07.10.1007/s00784-003-0208-512743837 [Google Scholar] [CrossRef] [PubMed]
[65]. Sculley DV, Langley-Evans SC, Periodontal disease is associated with lower antioxidant capacity in whole saliva and evidence of increased protein oxidationClin Sci 2003 105(2):167-72.10.1042/CS2003003112650638 [Google Scholar] [CrossRef] [PubMed]
[66]. Pavlica Z, Petelin M, Nemec A, Erzen D, Skaleric U, Measurement of total antioxidant capacity in gingival crevicular fluid and serum in dogs with periodontal diseaseAm J Vet Res 2004 65(11):1584-88.10.2460/ajvr.2004.65.158415566099 [Google Scholar] [CrossRef] [PubMed]
[67]. Lee MJ, Lambert JD, Prabhu S, Meng X, Lu H, Maliakal P, Delivery of tea polyphenols to the oral cavity by green tea leaves and black tea extractCancer Epidemiol Biomarkers Prev 2004 13(1):132-37.10.1158/1055-9965.EPI-03-004014744744 [Google Scholar] [CrossRef] [PubMed]
[68]. Amarnath K, Nellore J, Antioxidant potential of phytonanoparticles in an emerging biofluid (saliva) of periodontitis patientsNanoscience, Engineering and Technology (ICONSET), 2011 International Conference on 2011 IEEE10.1109/ICONSET.2011.6167989 [Google Scholar] [CrossRef]
[69]. Sakanaka S, Okada Y, Inhibitory effects of green tea polyphenols on the production of a virulence factor of the periodontal-disease-causing anaerobic bacterium Porphyromonas gingivalisJ Agric Food Chem 2004 52(6):1688-92.10.1021/jf030281515030231 [Google Scholar] [CrossRef] [PubMed]
[70]. Masaki K, Losonczy K, Izmirlian G, Foley D, Ross G, Petrovitch H, Association of vitamin E and C supplement use with cognitive function and dementia in elderly menNeurology 2000 54(6):1265-72.10.1212/WNL.54.6.126510746596 [Google Scholar] [CrossRef] [PubMed]
[71]. Rigotti A, Absorption, transport, and tissue delivery of vitamin EMol Aspects Med 2007 28(5):423-36.10.1016/j.mam.2007.01.00217320165 [Google Scholar] [CrossRef] [PubMed]
[72]. Hacquebard M, Carpentier YA, Vitamin E: absorption, plasma transport and cell uptakeCurr Opin Clin Nutr Metab Care 2005 8(2):133-38.10.1097/00075197-200503000-0000515716790 [Google Scholar] [CrossRef] [PubMed]
[73]. Traber MG, Vitamin E inadequacy in humans: causes and consequencesAdvances in Nutrition: An International Review Journal 2014 5(5):503-14.10.3945/an.114.006254PMC4188222 [Google Scholar] [CrossRef] [PubMed]
[74]. Linster CL, Van Schaftingen E, Vitamin CFEBS J 2007 274(1):01-22.10.1111/j.1742-4658.2006.05607.x17222174 [Google Scholar] [CrossRef] [PubMed]
[75]. Chan AC, Partners in defense, vitamin E and vitamin CCan J Physiol Pharmacol 1993 71(9):725-31.10.1139/y93-1098313238 [Google Scholar] [CrossRef] [PubMed]
[76]. Harrison FE, May JM, Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2Free Radic Biol Med 2009 46(6):719-30.10.1016/j.freeradbiomed.2008.12.01819162177 [Google Scholar] [CrossRef] [PubMed]