Nutrition plays a vital role in the growth and neurocognitive development of the offspring. Choline and DHA are essential brain nutrients and studies show that each nutrient has remarkable role on foetal mental development and neurocognitive outcomes throughout the lifespan. Nutrition is important because of the way in which it affects development. Nutrients play a critical role in, DNA synthesis, neurotransmitter and hormone metabolism, cell proliferation and are important contents of enzyme systems in the brain [1]. The effect of nutrition on the brain begins before birth which is associated with the nutrition of the mother during the prenatal period. Undernutrition during pregnancy and subsequent two years of life may cause adverse effects on brain development which may be permanent and irreversible [2].
National Academy of Sciences (NAS), United States identified choline as an essential nutrient for humans in 1998. Plasma concentration of choline has a physiological range of 10-50 μM. Choline is a quaternary amine (trimethyl-ß-hydroxy-ethylammonium). Choline utilisation is important during the synthesis of essential lipid components (phospholipids) present in the cell membranes [3]. The best sources of dietary choline are eggs and organ meat especially liver, soya lecithin, wheat germ and kidney beans. Other meats, dairy products, and bread are also significant dietary sources for most people. Food and Nutrition Board of the Institute of Medicine, US has estimated an Adequate Intake (AI) of choline of 550 mg/day for men and 425 mg/day for women in 1998 [4]. Animal experiments show that choline supplementation during gestation has a positive effect on memory functions of their offspring’s, this will be reflected on to the behaviour of animal for the entire lifespan. It has been proved that prenatal supplementation of choline protect normally observed memory decline even in old age [5].
Another important essential dietary nutrient is DHA, the most abundant polyunsaturated fatty acid present in the amino phospholipids of cell membranes, especially in the brain. Numerous studies have suggested that high concentration of DHA in the nervous system during development is necessary for optimal functions of the neurons and retina [6]. Recent animal studies proved that early DHA exposure has an influence on neurotransmitter systems, particularly dopamine, serotonin, choline, and y-amino butyric acid [7]. Prenatal foetal development during the last trimester and the postnatal development up to two years of after birth, the brain undergoes a rapid growth called as “brain growth spurt”. A considerable number of studies in both humans and animals have shown that DHA is one of the important nutrients required not only for sensory and cognitive development, but also plays an important role in the development of motor function of the nervous system during the brain growth spurt [8,9]. Various animal and human studies have reported that neural cell membranes require adequate levels of DHA for the maturation of cortical astrocyte, vascular coupling and for glucose uptake and metabolism in the neurons [10,11].
Although, essential nutrients like iron, calcium, and vitamins are supplemented during pregnancy, very few studies have focussed on essential brain nutrients and their role in the foetal mental development and neurocognitive outcomes through ageing. Moreover, there are no studies elucidating the comparative efficacy of supplementation of the essential brain nutrients choline or DHA alone and their synergistic supplementation prenatally on neurocognitive developmental outcomes in early adolescence.
In the present study, we aimed to assess the differences in neurocognitive developmental outcomes of early adolescent rats prenatally supplemented with either choline or DHA alone and compare the same with synergetic supplementation of both nutrients.
Materials and Methods
This animal experimental study was conducted in Kasturba Medical College, Manipal between October to December 2015.
Animals
Three months old adult female and male rats of Wistar strain (female 10 and male 5) were procured from Central Animal Research Facility, Manipal Academy of Higher Education, Manipal. These animals were nurtured in cages made of polypropylene and paddy husk was provided for the better bedding experience and retains in normal environments, 12:12 hour light and dark cycle, with free access to food and water. Prior approval was obtained from the Institutional Animal Ethics Committee (IAEC/KMC/32/2012) for this study. All the experiments using these animals were conducted to comply with the guidelines laid down by the CPCSEA Government of India. As rodents are nocturnal animals, all the behavioural tests were carried out in a dark room with the proper sound proof condition during the night between 7-10 p.m.
Study Design
Female rats in oestrus cycle were identified and allowed for mating with male rats at 2:1 ratio. The first day of gestation was determined by the vaginal smear test. Once pregnancy was confirmed (Embryonic day 0-E0) pregnant female rats were isolated and provided with nesting material and allowed to litter naturally. E0 Day pregnant dams (n=2/group) were divided into following five groups randomly. Group 1: Normal control (NC), Group 2: Saline control (SC), Group 3: Choline (C) supplemented, Group 4: DHA (DHA) supplemented, Group 5: Choline and DHA (C+DHA) supplemented. Subsequent to delivery, rat pups from each group were collected (note that each rat delivered ~ 10 to 12 pups) and after weaning these adolescent rats between Postnatal Day (PND) 30 to 40 (n=10/group) of both sexes were used for the study.
Dosage of Choline and DHA
Pregnant dams from NC were undisturbed in their home cage and were provided normal animal feed ad libitum. Pregnant dams from SC were supplemented with saline from E0 to delivery. Pregnant dams from C group were supplemented with Choline (Choline chloride 98% extra pure obtained from loba chemie laboratory reagents and fine chemicals) dissolved with distilled water 4.6 mmol/kg/day of Choline [12] from E0 to delivery. Pregnant dams from DHA group were supplemented with DHA (Soft Gelatin Capsule containing 400 mg Docosahexaenoic acid/capsule were obtained from Nouveau Medicament (P) Ltd., Chennai) as such from the capsules (400 mg/day of DHA) from E0 to delivery [13]. Pregnant dams from C+DHA were supplemented with both Choline and DHA from E0 to delivery. Subsequent to delivery adolescent rats from each group (n=10/group) were subjected to t-maze test for spatial learning and passive avoidance study between postnatal day 30th to 40th and the data were analysed in the statistical software Graph Pad Prism version 5.03.
Cognitive Assessment
Spatial learning test (T-maze): Rat pups aged PND 30-40 of both sex were subjected to spontaneous alternation test for spatial learning ability and rewarded alternation tests for memory retention on the T-maze as modified from Rai KS et al., and described by Bures J et al., [14,15]. All behavioural tests were conducted in a dark sound attenuated room.
Spontaneous alternation test [14,15]: Adolescent rats were starved for two days prior to the test in order to motivate them for a food reward. Subsequently, the pup’s body weight was maintained at 85% of pre test weight. Each rat was placed for 30 minutes daily, for two days, to orient them to the T-maze environment. During these sessions, 15 pellets of food were kept in each goal area. On the following four days, six trials were given daily with the food pellets in each goal area. The total number of alternations made by each rat was noted, and percentage bias was calculated.
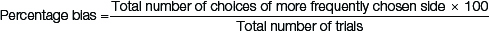
Rewarded alternation test [14,15]: This test was conducted on four consecutive days with six trials per day. In each trial, there were two runs, forced run and choice run. During the forced run, the rat was forced to enter the forced arm by blocking the other arm and was allowed to eat all the food pellets in the goal area. During the choice run, food pellet was placed in the goal area opposite to the forced arm, and there was no food pellet in the forced arm. Both the arms were open for the rat to enter. Now the rat had to choose and enter into the arm, where the pellet is placed. If the rat enters the arm, where the pellet is placed that is opposite to the forced arm, it is considered as “correct response”. The forced arm was same for all rats on any given day and it was pre decided. It was alternated on upcoming days. The experiment was repeated on four successive days and the “percentage of correct response” was calculated the following formula.
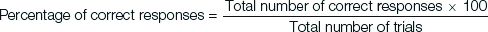
Passive avoidance test [14,15]: Briefly, on the first day, each adolescent rat was allowed to explore both the compartments of the passive avoidance apparatus for five minutes. On the second day, time spent in the large or small (dark) and the number of crossings between the large and small compartment were noted for each rat. After the learning session, the sliding door between the large and small compartments was closed, and the rat was cramped to the small compartment. Three unavoidable electric shocks (50 Hz, 1.5 mA, one second) was delivered to the rat on its foot. Then the rat was returned to its polypropylene cage. Memory retention test was performed 48 hours after foot shock. The rat was placed in the large compartment facing away from the small compartment then the sliding door between the large and small compartments was opened. The rat was free to explore both the compartments for about three minutes and after three minutes the rat was returned back to their homecage. The trial was repeated three times for each rat with an interval of five minutes between each trial. In each trial, the time spent in the smaller compartment and the number of crossings by each rat was recorded.
Statistical Analysis
Data are expressed as mean±SD and analysed with one-way ANOVA followed by Bonferroni’s multiple comparison tests to determine the significance between the groups using statistical software graph pad prism version 5.03. A p<0.05 is considered as significant.
Results
Spatial Learning and Memory
In this study, prenatal exposure to choline and DHA supplementation resulted in significant improvement in spatial learning abilities and cognitive functional outcomes during adolescence on the spontaneous and rewarded alternation test of the T-maze, as well as in the passive avoidance test.
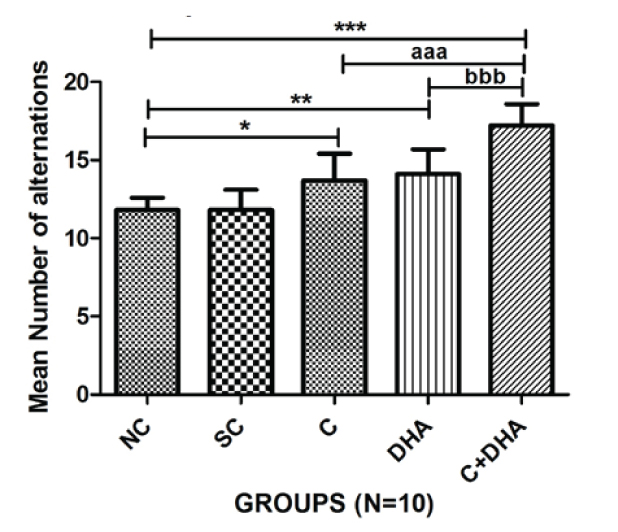
Postnatal day between 30-40 rats exposed prenatally to choline alone or DHA alone showed a significant number of alternations (*p<0.05 and **p<0.01) in spatial learning when compared with the same in age-matched normal control and saline control group of rats as shown in [Table/Fig-1]. Moreover, PND between 30-40 rats exposed prenatally to both choline and DHA showed significantly higher number of alternations (***p<0.001) in spatial learning when compared with the same in age-matched normal control and saline control group of rats. Additionally, significantly higher number of alternations (aaap<0.001 and bbbp<0.001) in spatial learning were observed in PND between 30-40 rats supplemented prenatally with both choline and DHA as compared to the same in age-matched rats supplemented either choline or DHA alone. No significant difference in performance on the spontaneous alternation test was observed between normal control and saline control groups of rats [Table/Fig-1].
T- Maze spontaneous alternation test: Mean number of alternations+Standard error in PND 30-40 rats. Normal control (NC), Saline control (SC), Choline (C), Docosahexaenoic acid (DHA) and Choline+Docosahexaenoic acid (C+DHA) age-matched group of rats.
One-way ANOVA followed by Bonferroni’s multiple comparison tests - *p<0.05, **p<0.01 and ***/aaa/bbbp<0.001.
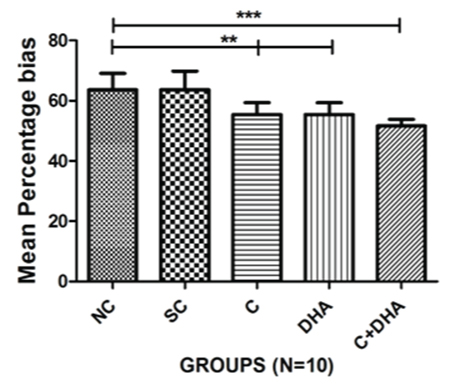
In the present study, PND between 30-40 rats supplemented prenatally with choline and DHA individually showed significantly less percentage bias when compared with age-matched normal control and saline control group of rats (*p<0.01). PND between 30-40 rats supplemented prenatally with choline and DHA showed significantly (***p<0.001) less percentage bias, when compared with age-matched normal control and saline control group of rats [Table/Fig-2].
T- Maze spontaneous alternation test: Mean percentage bias+Standard error in PND 30-40 rats. Normal control (NC), Saline control (SC), Choline (C), Docosahexaenoic acid (DHA) and Choline+Docosahexaenoic acid (C+DHA) age-matched group of rats.
One-way ANOVA followed by Bonferroni’s multiple comparison tests-**p<0.01 and ***p<0.001.
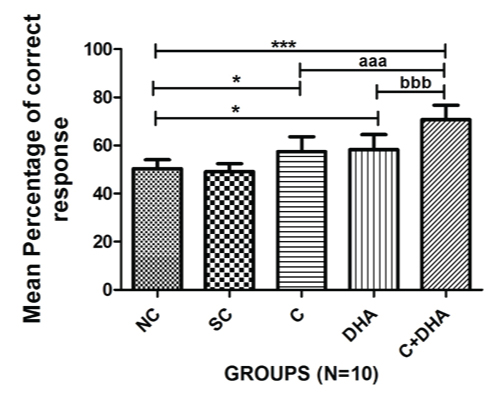
Postnatal day between 30-40 rats supplemented prenatally with choline and DHA individually shows a significant increase (*p<0.05) in the percentage of correct response when compared with age-matched normal control and saline control group of rats. PND between 30-40 rats supplemented prenatally with choline and DHA showed a highly significant increase (***p<0.001) in the percentage of correct response when compared with age-matched normal control and saline control group of rats. In addition, PND between 30-40 rats supplemented prenatally with choline and DHA, showed a highly significant increase (***p<0.001) in the percentage of correct response when compared with age-matched choline alone supplemented group (***p<0.001) and DHA alone supplemented (***p<0.001) group of animals. No significant difference in performance on the spontaneous alternation test was observed between normal control and saline control groups of rats [Table/Fig-3].
T- Maze rewarded alternation test: Mean percentage of correct response+Standard error in PND 30-40 rats. Normal control (NC), Saline control (SC), Choline (C), Docosahexaenoic acid (DHA) and Choline+Docosahexaenoic acid (C+DHA) age-matched group of rats.
One-way ANOVA followed by Bonferroni’s multiple comparison tests-
*p<0.05, and ***/aaa/bbbp<0.001.
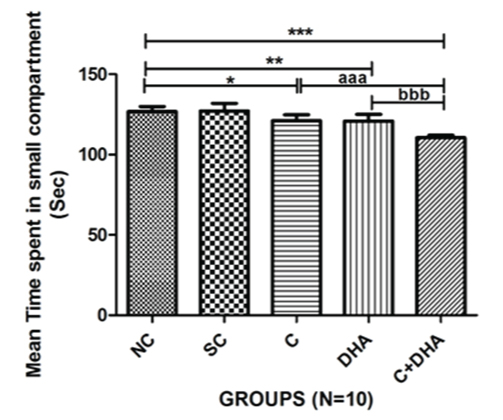
Passive Avoidance Test
Exploration: During exploration, there were no significant differences observed among the groups and for the time spent in the dark compartment.
Retention: PND between 30-40 rats supplemented prenatally with choline and DHA individually stayed significantly less time in small (dark) compartment choline supplemented group (**p<0.05), DHA supplemented group (**p<0.01) and PND between 30-40 rats supplemented prenatally with choline and DHA spent significantly (***p<0.001) very less time in the small compartment which is highly significant. PND between 30-40 rats supplemented prenatally with choline and DHA spent less time in the small compartment when compared with age-matched choline alone supplemented group of rats (***p<0.001) and DHA alone supplemented group of rats (***p<0.001) which is also highly significant [Table/Fig-4].
Meantime spent in small compartment+Standard error in PND 30-40 rats. Normal control (NC), Saline control (SC), Choline (C), Docosahexaenoic acid (DHA) and Choline+Docosahexaenoic acid (C+DHA) age-matched group of rats.
One-way ANOVA followed by Bonferroni’s multiple comparison tests-
* p<0.05, **p<0.01 and ***/aaa/bbb p<0.001.
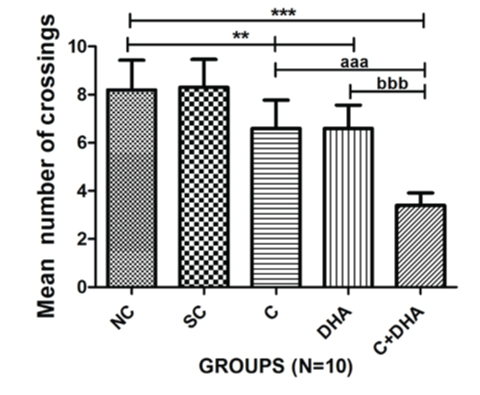
Postnatal day between 30-40 rats supplemented prenatally with choline and DHA individually showed significantly (**p<0.01) less number of crossing in retention test and PND between 30-40 rats supplemented prenatally with choline and DHA showed highly decreased in the number of crossing (***p<0.001). PND between 30-40 rats supplemented prenatally with choline and DHA showed less number of crossings when compared with age-matched choline alone supplemented group of rats (***p<0.001) and DHA alone supplemented a group of rats (***p<0.001) group of animals which is also highly significant [Table/Fig-5].
Mean number of crossings+Standard error in PND 30-40 rats. Normal control (NC), Saline control (SC), Choline (C), Docosahexaenoic acid (DHA) and Choline+Docosahexaenoic acid (C+DHA) age-matched group of rats.
One-way ANOVA followed by Bonferroni’s multiple comparison tests-
**p<0.01 and ***/aaa/bbb p<0.001.
Discussion
Earlier studies have shown some beneficial effects of choline and DHA independently on brain development and cognitive functions in adults [16-19]. In this study, the efficiency of either or both choline and DHA supplementation on the neurocognitive development of offspring were evaluated. PND 30-40 rats supplemented prenatally with choline and DHA individually showed significant enhancement in spatial learning when compared to the same in age-matched normal control and saline control group of animals. Additionally, this group of animals also showed significantly more number of alternations and percentage of correct response as well as less percentage bias. Studies show that higher levels of choline administered during embryonic days results in enhanced spatial and temporal memory of offspring adult rats throughout their life span [5]. Choline supplementation during gestation enhances Brain Derived Neurotrophic Factor (BDNF) and Nerve Growth Factor (NGF) which leads to increase in neurogenesis, hippocampal plasticity of their offspring, thereby influencing learning and memory function [20]. Choline has a positive influence on stem cell proliferation and apoptosis of neural cells especially during foetal development, thus influencing the development of neural tube and brain, thus influencing memory function [3]. DHA supplementation has been associated with improved scores of memory and learning capabilities in rats when evaluated through the Morris water maze test and radial 12 arms maze test [21,22]. Other studies have shown that DHA supplementation increases growth cone of neurite outgrowth, and synaptosomes that coincide with the onset of synaptogenesis which is an important feature during neurodevelopment [23].
Results from passive avoidance test also show that PND 30-40 rats supplemented prenatally with choline and DHA individually, spent significantly less time in the small compartment and less number of crossing in retention test when compared to the same in age-matched normal control and saline control group of animals. Results of this study are consistent with an earlier study showing that choline and DHA supplementation in the form of DHA-LPC, increases the learning and memory capabilities of pups from mothers that received the supplement during the perinatal period [24]. Maternal supplementation with a long-chain polyunsaturated fatty acid like DHA during pregnancy and lactation may increase children’s intelligence [25]. DHA improves spatial learning and memory impairment in rats treated with propofol anaesthesia by enhancing the activity of antioxidant enzymes, balancing the levels of acid neurotransmitters and increasing the concentration levels of BDNF [26].
However, PND 30-40 rats supplemented prenatally with both choline and DHA showed significantly more improvement in spatial learning when compared to the same in not only age-matched normal control and saline control group but also in those rats supplemented choline or DHA alone. No significant difference in performance on the spontaneous alternation test was observed between normal control and saline control groups of rats. Similarly, in passive avoidance test, PND 30-40 rats supplemented prenatally with both choline and DHA showed significantly more memory retention, spending less time in small (dark) compartment and also less number of crossings between the large and small compartments when compared to the same in not only age-matched normal control and saline control group of rats but also in those rats supplemented choline or DHA alone. Thus, availability of both choline and DHA simultaneously during neurodevelopment allows for increased laying down of membranes a prerequisite for increased neural cell formation in brain especially in the hippocampus and dentate gyrus which helps in better enhancement of learning and memory. Studies show that metabolism of choline and DHA are linked by the enzyme Phosphatidylethanolamine-N-Methyltransferase (PEMT) which catalyses de novo biosynthesis of Phosphatidylcholine (PtdCho) by methylation of Phosphatidylethanolamine (PtdEtn) enriched with DHA [27,28]. This enzyme prefers species of PtdEtn that contain long-chain polyunsaturated fatty acids such as DHA thereby, forming DHA-enriched species of PtdCho in membranes, especially in the brain. Available PtdCho and PtdEtn are also used to form Phosphatidylserine (PtdSer) by serine base exchange enzymes [29]. This may be the reason why combined administration of choline and DHA showed significantly better enhancement in spatial learning and memory in T-maze as well as avoidance memory retention in passive avoidance tests compared to those supplemented with these nutrients individually.
Limitation
Study was limited to early adolescent period. The study shows only the cognitive outcomes in this particular age group. The mechanisms for the alterations in cognition need to be evaluated.
Conclusion
Combined supplementation of choline and DHA during prenatal period significantly enhances neurocognitive developmental outcomes on early adolescent learning and memory than supplementing these nutrients separately.