Cholecystectomy specimens are one of the most common surgically resected materials at many surgical pathology laboratories [1,2] paralleling the prevalence of clinically evident and asymptomatic gallbladder disorders. Most common histologically identified lesion of gallbladder is chronic cholecystitis which on most occasions is associated with cholelithiasis in 78-90% cases [3,4] and not uncommonly an unsuspected malignancy is discovered only on histopathological examination which is regarded as gold standard [5].
Cholelithiasis is the most common gastrointestinal disease exerting significant burden on healthcare system [6]. It is the most common indication for cholecystectomy and also the most common associated factor for carcinoma gallbladder irrespective of age and sex [7]. Wide varieties of mucosal changes are identified in both clinically symptomatic and asymptomatic gallbladder specimens. Some of these epithelial changes at times form precursor lesion and thus, are associated with increased risk for gallbladder malignancies [8] hence, histopathological recognition and further study including the genetic basis of such lesions may improve our understanding regarding the aetiopathogenesis of these cancers.
Wide variety of gallbladder disorders are diagnosed histologically which include inflammatory lesions, metaplastic changes, hyperplasia, dysplasia, adenoma, and carcinoma [1,2]. Incidental detection of gallbladder malignancy on microscopic examination is the clinically most significant information for the surgeon as well as for the patient and this fact emphasises the importance of subjecting each gallbladder specimen for histopathological examination [5]. Many cases have been published in literature suggesting that cases where cholecystectomy was done for gallstone disease and where microscopic examination was omitted, later presented with hepatic metastasis from unknown primary origin [9]. Hence, histopathological examination in all cases of cholecystectomy is essential. The present study was undertaken with following aims to study: (1) morphological spectrum of gallstone disease in rural population of Northern India, (2) correlation of cholelithiasis with benign and malignant lesions.
Materials and Methods
This retrospective study was undertaken in a Tertiary Care Hospital and Medical College to identify various histologic alterations occurring in the gallbladder mucosa associated with or without cholelithiasis. A total of 1693 resected specimens of gallbladder received during January 2012 to June 2016 were included. Specimens which were not sent in proper fixative and where morphological details were not discernible were excluded. Clinical details and other relevant information were collected from the records including gross examination findings-size of gallbladder, serosa, wall thickness, mucosa, presence/absence of stones, their number, and type was noted along with any mass lesions. At least three representative sections were taken, one each from fundus, body and neck, additional sections were taken whenever any grossly visible abnormal area was present. Microscopic examination was performed on formalin fixed paraffin embedded haematoxylin and eosin stained tissue sections where following features were assessed-inflammation, cholesterolosis, granulomas, metaplasia, calcification, dysplasia, benign and malignant neoplasms. Ethical clearance was obtained from Institutional Ethical Committee.
Statistical Analysis
Statistical correlation was calculated using chi-square test and p-value was obtained. Value of p<0.05 was considered statistically significant.
Results
In the present series a total of 1693 cholecystectomies were subjected to histopathologic evaluation. There were 738 males (43.6%) and 955 females (56.4%). Most of the cases were of age group 31-40 years (30%) and only two cases (both malignant) occurred in age group of 81-90 years. Majority of malignant cases occurred in the eighth decade. Youngest male and female affected in the series were 10 and 12 years respectively with the diagnosis of chronic cholecystitis and youngest case of malignancy was in third decade [Table/Fig-1]. Various types of lesions diagnosed in the series and their relation with number of stones as shown in [Table/Fig-2]. Most common diagnosis rendered was chronic cholecystitis accounting for 69.1% cases and was relatively more common in females. Majority of the lesions were more common in females except for the porcelain gallbladder [Table/Fig-3], dysplasia, adenoma, and carcinoma that showed significant preponderance for males with M:F ratio of 3:1, 4:1, 2.5:1, and 7:1 respectively.
Histology distribution with age.
| Age (in years) | No. of benign cases | No. of malignant cases | Total no. of cases |
|---|
| <20 | 79 | 0 | 79 |
| 21-30 | 457 | 1 | 458 |
| 31-40 | 505 | 0 | 505 |
| 41-50 | 332 | 1 | 333 |
| 51-60 | 203 | 2 | 205 |
| 61-70 | 89 | 4 | 93 |
| 71-80 | 12 | 6 | 18 |
| 81-90 | 0 | 2 | 2 |
| Total | 1677 | 16 | 1693 |
Distribution of various lesions of gallbladder and their relation with number of stones (n=1693).
| Lesions | No. of cases (%) | Number of stones |
|---|
| Single (%) | Multiple (%) |
|---|
| Chronic cholecystitis, N=1170 (69.1%) |
| Chronic cholecystitis alone | 265 (15.7) | 51 (19.2) | 214 (80.8) |
| Chronic cholecystitis with Cholesterolosis | 308 (18.2) | 247 (80.2) | 61 (19.8) |
| Chronic cholecystitis with Metaplasias | 439 (25.9) | 108 (24.6) | 331 (75.4) |
| Chronic cholecystitis with hyperplasia | 64 (3.78) | 36 (56.3) | 28 (43.7) |
| Chronic cholecystitis with adenomayomatosis | 88 (5.2) | 31 (35.2) | 57 (64.8) |
| Chronic cholecystitis with ceroid granuloma | 6 (0.35) | 01 (16.6) | 05 (83.3) |
| Cholecystitis various types, N=478 (28.2%) |
| Acalculous cholecystitis | 68 (4.01) | - | - |
| Acute/chronic cholecystitis | 195 (11.5) | 93 (47.7) | 102 (52.3) |
| Chronic active cholecystitis | 106 (6.2) | 45 (42.5) | 61 (57.5) |
| Xanthogranulomatous cholecystitis | 34 (2.1) | 06 (17.6) | 28 (82.4) |
| Follicular cholecystitis | 41 (2.4) | 11 (26.8) | 30 (73.2) |
| Eosinophilic cholecystitis | 14 (0.82) | 02 (14.3) | 12 (85.7) |
| Granulomatous cholecystitis | 2 (0.11) | 0 (0) | 02 (100) |
| Empyema | 6 (0.35) | 0 (0) | 06 (100) |
| Porcelain gallbladder | 12 (0.7) | 02 (16.7) | 10 (83.3) |
| Benign, premalignant and malignant lesions, N=45 (2.7%) |
| Dysplasia | 15 (0.88) | 06 (40) | 09 (60) |
| Adenoma | 14 (0.82) | 03 (21.4) | 11 (78.6) |
| Carcinoma | 16 (0.94) | 03 (18.8) | 13 (81.3) |
| Total | 1693 | 645 (38.2) | 980 (57.8) |
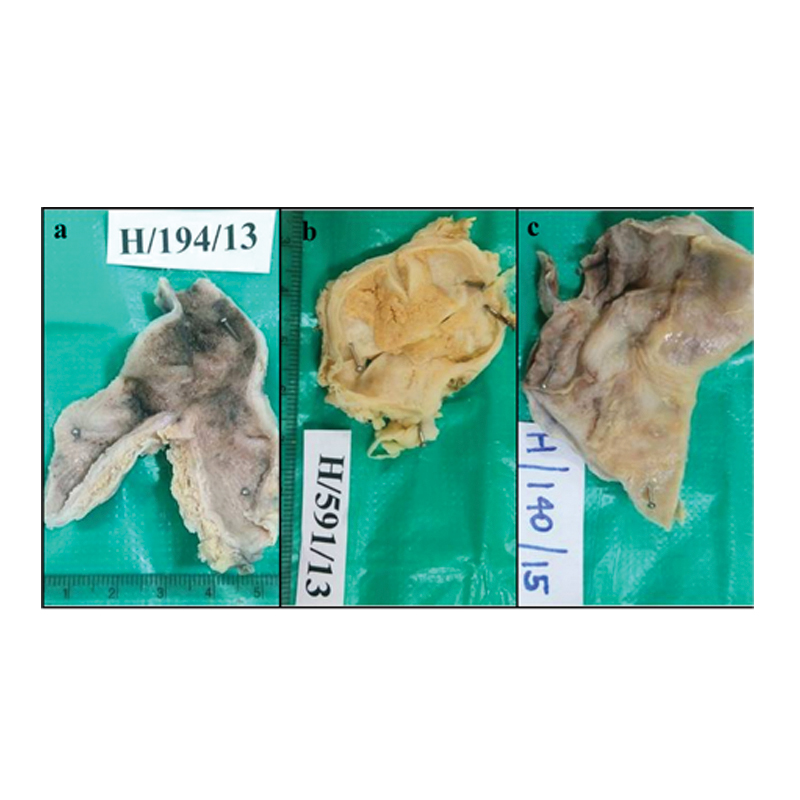
a) Gross photograph showing thick wall gallbladder corresponding to porcelain gallbladder; b) Gross photograph showing thick wall gallbladder with yellow areas corresponding to xanthogranulomatous cholecystitis; c) Gross photograph showing grey white and necrotic areas corresponding to granulomatous cholecystitis.
Stone characteristics and its association with various benign and malignant cases are shown in [Table/Fig-4]. There was associated cholelithiasis in 1609 benign and 16 malignant lesions. Most common type of stone was mixed, present in 64.6% cases. No stones were present in 68 cases (4.01%). All these acalculous cases showed features of chronic cholecystitis. Total of 643 benign and 03 malignant cases had single stone and 966 benign and 13 malignant cases had multiple stones. All the cases of granulomatous cholecystitis (02) and empyema (06) had multiple stones. A statistically significant relation was found between presence of stones and carcinoma (p=0.01). However, no significant correlation was found between presence of stones and benign lesions (p=0.416).
Stones characteristics and its association with benign and malignant cases.
| S. No | Type of stone | Description | No. of cases (%) | Single | Multiple |
|---|
| Benign (%) | Malignant (%) | Total | Benign (%) | Malignant (%) | Total |
|---|
| 1. | Cholesterol | Solitary, oval, large, granular, yellow white. Cut section: Radiating crystals. | 382 (23.51%) | 379 (99.2) | 03 (0.8) | 382 (100%) | 0 (0) | 0 (0) | 0 (0) |
| 2. | Pigment | Multiple, small, jet black. Cut section: Black. | 104 (6.4%) | 22 (100) | 0 (0) | 22 (40.4) | 72 (87.8) | 10 (12.2) | 82 (59.6) |
| 3. | Mixed | Multiple, multifaceted. Cut section: Alternate dark and pale white areas. | 1050 (64.6%) | 158 (100) | 0 (0) | 158 (14.1%) | 889 (99.7) | 03 (0.35) | 892 (85.9%) |
| 4. | Combined | Single, large smooth. Cut section: Central nucleus of pure cholesterol with mixed shell or vice versa. | 89 (5.48%) | 84 (100) | 0 | 84 (83.1%) | 05 (100) | 0 | 05 (16.9%) |
| Total | | 1625 | 643 | 03 | 646 | 966 | 13 | 979 |
Details of selected lesions diagnosed in the series and their stone characteristics are given in [Table/Fig-5]. Amongst 439 cases showing metaplasia, antral type metaplasia [Table/Fig-6a] (186 (42%)) was most common followed by intestinal metaplasia (102 (23%)). Other types of metaplasia seen were goblet cell, paneth cell [Table/Fig-6b] and squamous metaplasia. Majority of the cases with metaplasia had multiple mixed stones. Amongst 64 cases of hyperplasia, most common was adenomatous hyperplasia (31.3%) followed by papillary hyperplasia (68.7%). Most common stone associated with hyperplasia was mixed type seen in 41 cases. Dysplasia was observed in only 0.88% cases and most common stone type was multiple mixed (07 cases) followed by single cholesterol (05 cases). Most frequent stromal change was cholesterolosis (18.2%) [Table/Fig-6c] which was frequently associated with single stone of cholesterol type however, multiple mixed stones were also present. Next most common stromal change was adenomyomatosis (5.2%) [Table/Fig-6d] followed by follicular cholecystitis [Table/Fig-7a] and eosinophilic cholecystitis [Table/Fig-7b] was rare. Latter two lesions were associated with multiple mixed stones.
Association of various lesions with type and number of stones.
| Lesion | No. of cases (%) | Cholesterol | Mixed | Pigment | Combined |
|---|
| Chronic cholecystitis with Metaplasias | 439 (25.9) | 80 (S) | 26 (S) 303 (M) | 28 (M) | 02 (S) |
| Chronic cholecystitis with Hyperplasia | 64 (3.78) | 08 (S) | 21 (S) 20 (M) | 04 (M) | 07 (S) 04 (M) |
| Xanthogranulomatous cholecystitis | 34 (2.1) | 05 (S) | 25 (M) | 03 (M) | 01 (S) |
| Follicular cholecystitis | 41(2.4) | 11 (S) | 30 (M) | - | - |
| Eosinophilic cholecystitis | 14 (0.82) | 01 (S) | 09 (M) | 03 (M) | 01 (S) |
| Porcelain GB | 12 (0.7) | 02 (S) | 10 (M) | - | - |
| Dysplasia | 15 (0.88) | 05 (S) | 07 (M) | 02 (M) | 01 (S) |
| Adenoma | 14 (0.82) | 03 (S) | 11 (M) | - | - |
| Carcinoma | 16 (0.94) | 03 (S) | 03 (M) | 10 (M) | - |
(S)= Single, (M)= Multiple
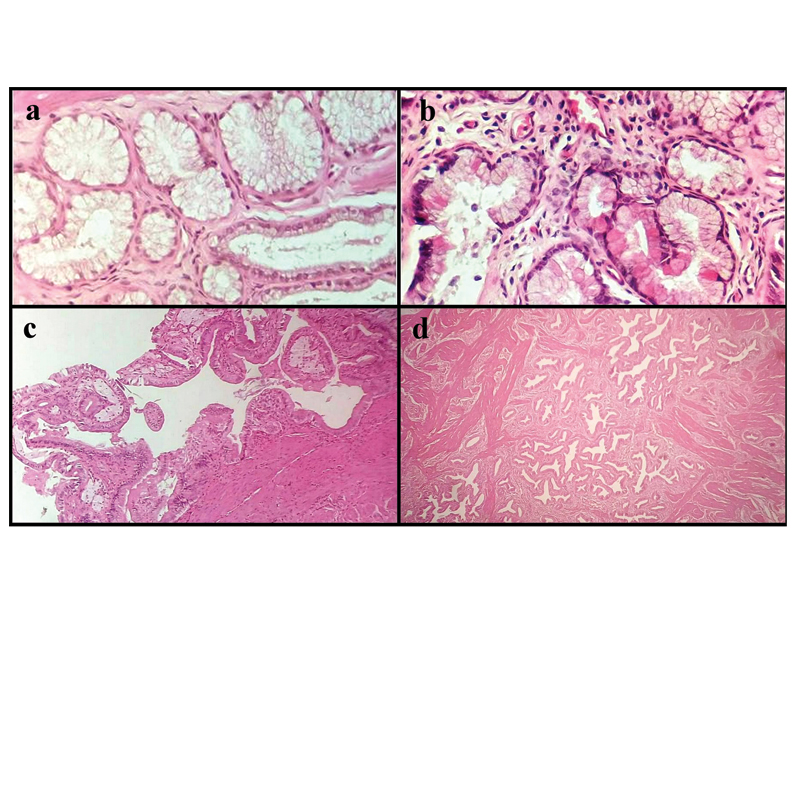
a) Section showing pyloric metaplasia (H&E, 40X); b) Section showing paneth cell metaplasia with cells showing eosinophilic granular cytoplasm (H&E, 40X); c) Section showing foamy macrophages in the mucosa representing cholesterolosis (H&E, 10X); d) Section showing hypertrophy of muscularis layer with hyperplasia of glands representing adenomyomatosis (H&E, 10X).
a) Section showing lymphoid follicles in mucosa and submucosa representing follicular cholecystitis (H&E, 10X); b) Section showing eosinophilic cholecystitis (H&E, 40X); c) Section showing epithelioid cell granulomas with langhans giant cells and necrosis in gallbladder (H&E, 20X); d) Section showing ceroid granulomas (H&E, 40X).
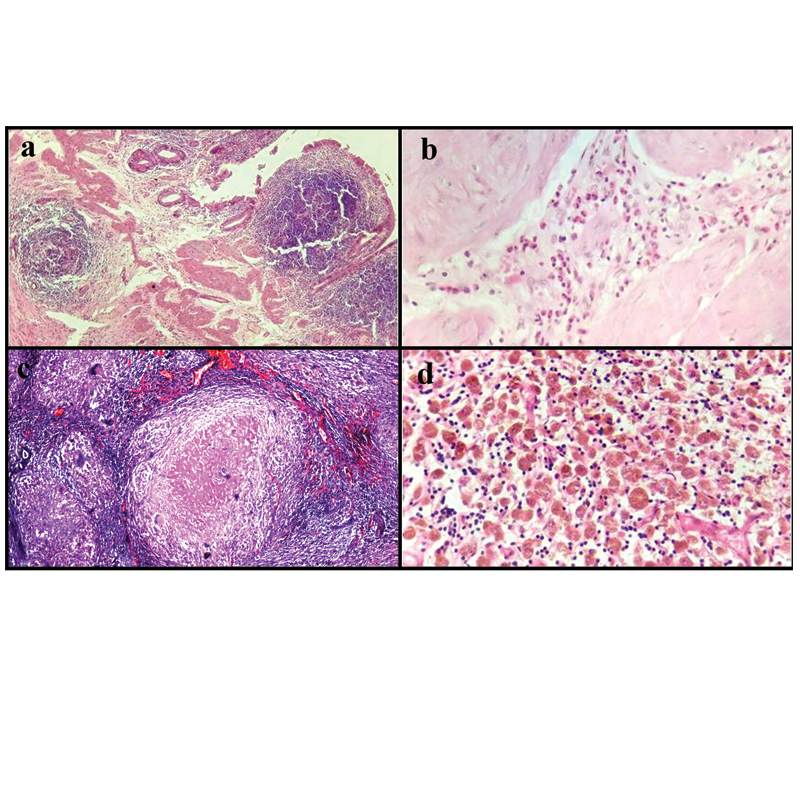
Xanthogranulomatous [Table/Fig-3b] and granulomatous [Table/Fig-7c,3c] cholecystitis accounted for 2.1% and 0.11% cases respectively with the latter being the rarest type of lesion in the present series. Ceroid granuloma [Table/Fig-7d] was seen in 6 (0.35%) of cases.
Twelve (0.7%) cases of porcelain gallbladder [Table/Fig-8a] were also observed where there were intramural (08 cases) and mucosal (04 cases) calcification along with paucicellular hyaline fibrosis. All cases had cholelithiasis with majority (10 cases) showing multiple mixed stones. None of the case had carcinoma.
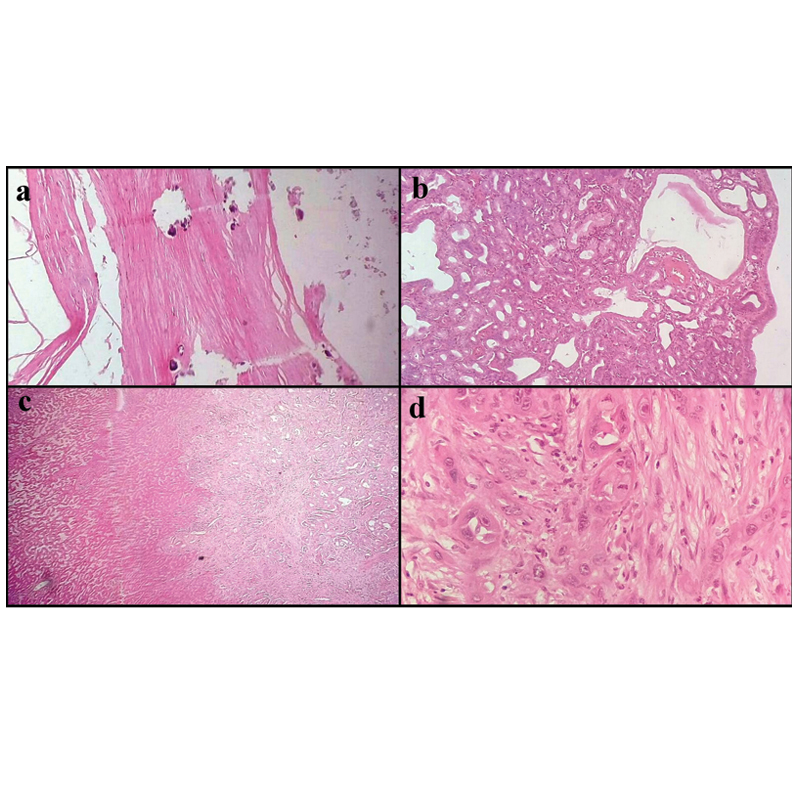
Amongst 14 cases of adenoma, 08 cases were of tubular adenoma [Table/Fig-8b], 04 were of tubulovillous and 02 of villous adenoma. In 11 cases multiple mixed stones were present. Of the 16 cases of carcinomas, moderately differentiated adenocarcinoma [Table/Fig-8c], was the most common gallbladder malignancy seen in 10 cases. Two cases of poorly differentiated carcinoma were reported [Table/Fig-8d]. One case each of signet ring cell and mucinous adenocarcinoma was also observed. Association between cholelithiasis and gallbladder carcinoma was 100% (16 cases) and the multiple pigment stone was found in 10 cases (62.5%). In remaining cases, three had single cholesterol and three had multiple mixed stones [Table/Fig-9].
a) Section showing calcification in wall of gallbladder representing porcelain gallbladder (H&E, 10X); b) Section showing tubular adenoma (H&E, 10X); c) Section showing moderately differentiated adenocarcinoma infiltrating liver (H&E, 4X); d) Section showing poorly differentiated adenocarcinoma (H&E, 40X).
Types of adenocarcinoma and its association with type and number of stones.
| Types of carcinoma | Mucinous | Signet ring | Papillary | Moderately differentiated | Poorly differentiated |
|---|
| No. of cases | 1 | 1 | 2 | 10 | 2 |
| Stone | 01 Multiple mixed | 01 Multiple mixed | 01 Multiple mixed, 01 Multiple pigment | 03 single Cholesterol, 07 Multiple pigment | 02 Multiple pigment |
Discussion
In India, the documented prevalence of gallstone disease varies between (2-29%) and is seven times more common in Northern compared to Southern India [1]. Gallbladder is known to show various mucosal changes including hyperplasia, metaplasia and dysplasia in response to stones [1,5].
In the present study, maximum numbers of cases were between 31 to 40 years similar to an Indian study conducted by Mohan H et al., and Vani BR et al., [1,5]. Chronic cholecystitis was the most common lesion seen in 69.1% cases and most commonly found in females. Associated calculi was present in 1625 cases, mixed and cholesterol type accounting for 64.6% and 23.51% respectively, based on morphological appearance. This is in concordance with study by Mohan H et al., where 62% were mixed stones. Mixed stones are the most common type encountered in Northern India [1]. In our study too, maximum number of cases had mixed stones.
All carcinoma cases were associated with calculi and maximum cases showed pigment stones. According to study by Mohan H et al., and Jain BB et al., [1,10] carcinomas are significantly associated with cholelithiasis and this correlation was also seen in our study. Martinez-Guzman G et al., concluded in their study that low and high grade dysplasia, tubular adenomas, carcinoma in situ and invasive carcinoma were more frequent when cholelithiasis was present (p<0.05) as compared to cases without gallstones [11]. A study conducted by Vitetta L et al., showed that primary carcinoma of gallbladder was always associated with single or multiple cholesterol gallstones that were seen impacting on gallbladder wall [12]. Association between cholelithiasis and gallbladder carcinoma has been reported in 40-100% cases [7] and it was 100% in the present series.
In 0.5 to 1.1% cases incidental gallbladder malignancies may be detected on microscopy that were missed during pre and per operative examination [13-16]. Hence, several authors believe that all cholecystectomies must be subjected to gross and histopathological examination as even normal looking gallbladder may harbour significant pathologies [2,17-20] like dysplasia and early mucosal carcinomas, though in such cases simple cholecystectomy is regarded curative and no further intervention is needed [15,16,21,22]. However, histopathologic identification of carcinoma with perineural or transmural invasion carries dismal prognosis and re-emphasises upon the need to examine all cholesystectomies [5].
Early gallbladder carcinomas may closely mimic chronic cholecystitis as both show thickening of the gallbladder wall [23]. Rao I et al., [24] mentioned that gross identification of tumour in 10-37% cases of gallbladder carcinoma is uncertain and likely to be missed if not subjected to microscopy. In the present study, incidental gallbladder malignancy was found in 12 patients accounting for 0.7%. In all these 12 patients, there was macroscopic evidence of disease in the form of thickening of gallbladder wall or ulceration of mucosa. A case of signet ring cell adenocarcinoma was found, this variant is regarded as highly malignant tumor that carries worse prognosis [25].
In the present study, dysplasia was present in 15 (0.88%) cases where it was mostly associated with mixed stones. Close association between gallstones and dysplasia was also observed by Gupta SC et al., and Jain BB et al., [3,10]. Reported frequency of dysplasia in literature varies from 0.08% to 2.2% [5,26-28]. Mukuda T et al., [29] mentioned that precancerous lesions are frequently overlooked by the pathologists and it seems to be the most probable reason for very less frequency of dysplasia being reported in various series.
Xanthogranulomatous cholecystitis was reported in 34 (2.1%) cases. Its frequency in present series was comparable to that reported by Mohan H et al., [1]. Slightly higher frequency of 3.6% was reported by Khan S et al., [2]. An increased frequency of gallbladder carcinoma has been associated with xanthogranulomatous cholecystitis varying from 6 to 10% in different series [30,31]. In the present series no association between the two was observed. A series on xanthogranulomatous cholecystitis comprising of 33 cases was reported by Pandey P et al., [32] where on gross examination lesions mainly occurred in fundus or body region and presented as yellowish raised nodules. Most of the cases in this series were associated with cholelithiasis. In the present series also there was thickened gallbladder wall along with yellowish nodules located mainly in the fundus with 80% association with cholelithiasis predominantly of mixed type.
Granulomatous cholecystitis was observed in two cases where there were many epithelioid cell granulomas and multinucleated giant cells including langhan’s type. In one of the cases there was past history of abdominal tuberculosis and this case also showed caseous necrosis along with cholelithiasis but acid fast bacilli could not be demonstrated. Various causes of granulomatous cholecystitis include sarcoidosis [33], tuberculosis [34-36] and crohn’s disease [37]. Primary tuberculosis of gallbladder is extremely rare [34,35] as gallbladder mucosa is considered resistant to tuberculosis and moreover, cholelithiasis and cystic duct obstruction are regarded as the prerequisites for its development [36]. In another case cause of granulomatous inflammation could not be identified.
In the present series porcelain gallbladder was seen in 12 cases (0.7%) with 08 and 04 cases showing intramural and mucosal calcification respectively. All the cases were seen in sixth decades. It was three times more common in males compared to females. The extent of calcification varied from case to case with 04 cases showing complete calcification. Most of the cases were devoid of epithelium and association with cholelithiasis was 100%. The reported frequency of porcelain gallbladder is between 0.6 to 0.8% [38]. It is regarded as end stage of chronic inflammation and scar formation as supported by its occurrence in late decades. Its association with malignancy was believed to be more frequent in previous literature [10]. However, recent reports have shown lesser association between the two (04 to 07%) and moreover mucosal calcification is more closely related to carcinoma compared to intramural one [39]. In the present series none of the cases had carcinoma.
Amongst the stromal changes seen in gallbladder, most common was cholesterolosis seen in 18% cases. Most of these cases were associated with cholesterol stones. Follicular cholecystitits is a variant of chronic cholecystitis with lymphoid hyperplasia in the wall. It was seen in 41 cases (2.4%). Its frequency in various series varies from 2.3% to 0.5% [1,2].
In our study, 64 cases of hyperplasia were seen. Most common being adenomatous hyperplasia [17]. Elfving G et al., proposed the hypothesis that presence of calculi leads to chronic irritation that causes secondary hyperplasia [40].
Metaplasia is not an infrequent finding in gallbladder. Chronic cholecystitis with metaplasia was noted in 25.9% cases of our study. Likewise, pyloric metaplasia was more common than intestinal metaplasia. Our results were comparable to those reported by other studies with similar distribution of metaplasia cases [41, 42]. It is well documented that metaplastic mucosa is more likely to undergo neoplastic change compared to the normal mucosa [28]; moreover, intestinal metaplasia is believed to be a precancerous lesion [43].
Conclusion
The present study involved microscopic examination of 1693 cholecystectomy specimens and its correlation with presence of stones. Present study is the largest series conducted in the rural population in Northern India till date. Rare cases like porcelain gallbladder and granulomatous cholecystitis were also observed in our series. However, we encountered certain limitations like, ultrasonography details were not provided in all cases, and so radiological correlation was not possible. Follow up of patients with carcinoma was not done. Proper clinical history was not provided so correlation with cause of stone was not found like, cases of haemolytic anaemia generally present with pigment stones etc. Also biochemical examination of the stones were not done in our institute.
Since, gallbladder malignancy is detected incidentally in many cholecystectomy specimens, the need of detailed microscopic examination is emphasised in all cholecystectomies.
(S)= Single, (M)= Multiple