Colorectal cancer is an important public health problem and a major cause of morbidity and mortality throughout the world [1]. Of all the cases reported worldwide, incidence in developed world accounts for about 63% of cases [2].
In India, colon cancer ranks eighth and rectal cancer ranks ninth among men. In women, rectal cancer does not figure in top ten; whereas, colon cancer ranks ninth [3]. Global age standardised rates of colorectal carcinoma incidence are higher in men than in women (19.1/1,00,000 in men and 14.4/1,00,000 in women) [4].
Tumours require neovascularisation for growth and metastasis. VEGF is a signal protein that can induce angiogenesis and lymphangiogenesis [5]. It was discovered in 1983 by Senger DR et al., [6]. VEGF promotes regional and distant metastasis, thereby reducing survival [7]. Thus, assessing the expression of VEGF in colorectal malignancies can help in determining the prognosis and in improving patient survival.
In this study, an attempt has been made to study the expression of VEGF in colorectal malignancies and to compare it with various clinicopathological parameters and to determine the prognostic significance.
Materials and Methods
The study was combined prospective and retrospective, conducted over a period of two years from July 2013 to July 2015 after obtaining clearance from the Institutional Ethics Committee (No. 12102014). We received 147 cases of resected specimens of colorectal carcinomas for histopathological examination in Institute of Pathology, Madras Medical College. Colorectal malignancies diagnosed in resected specimens were included in the study. Small biopsies, conditions such as lymphoma of the colon, GIST, non neoplastic and benign lesions were excluded from the study.
Relevant clinical details of the patients undergoing surgery for colorectal malignancy were collected. The clinicopathological variables studied were age, gender, site of the tumour, size of the tumour, gross appearance, histological type, grading and staging of the tumour, other prognostic factors like lymphovascular invasion and presence of lymph node metastasis.
Four micron thick sections were taken from formalin fixed paraffin embedded tissue blocks and stained with haematoxylin and eosin. Among them, 50 random cases were selected for immunohistochemical studies. Immunohistochemical analysis was done using VEGF monoclonal antibodies. Sections showing features of pyogenic granuloma were used for control. The vascular endothelial cells showed cytoplasmic positivity for VEGF [Table/Fig-1].
Strong VEGF cytoplasmic positivity in the tumour cells (40X); Inset shows the VEGF positivity in the control tissue used (pyogenic granuloma), IHC (10X).
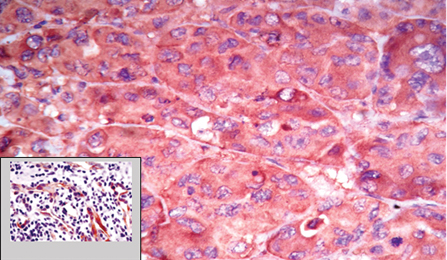
Interpretation and scoring system: The slides were analysed for the presence of immunohistochemical reaction, percentage of cells stained and intensity of the reaction. The slides were screened for cytoplasmic positivity of VEGF. Intensity of staining was classified as weak, moderate and strong [8].
Scoring was done based on the proportion of the cells stained.
0- no tumour cells show positivity
1- <10 tumour cells show cytoplasmic positivity
2- 11-50% cells show cytoplasmic positivity
3- >50% cells show cytoplasmic positivity
Statistical Analysis
The statistical analysis was done using Statistical Package for Social Science (SPSS) software - IBM SPSS version 20.0. The expression of VEGF was correlated with variables like age, gender, location, size, grade, stage, histological grade, lymph node involvement and lymphovascular invasion. Chi-square test was used to obtain the p-values (p-values <0.05 were considered significant).
Results
In this study, the median age at presentation of colorectal cancer was 60 years. The youngest age of presentation was 23 years and the oldest age of presentation was 79 years. Most of the cases were in the age range of 51 to 60 years (42.9%). Out of 147 cases, 85 (57.8%) were males and 62 (42.2) were females. A total of 104 (70.7%) cases had the primary tumour in the left colon and 43 (29.3%) cases in the right colon. The tumour size was <5 cm in 79 (53.7%) cases and >5 cm in 68 (46%) cases, 92 (62.6%) cases were ulceroproliferative [Table/Fig-2], 23 (15.6%) were ulcerative, 12 (8.2%) were ulceronodular, 14 (9.5%) were circumferential, 2 (1.4%) cases presented as stricture and 4 (2.7%) cases presented with polypoidal masses.
Gross appearance of a caecal growth.

Among the 147 cases, 123 (83.7%) were infiltrating adenocarcinoma [Table/Fig-3], 6 (4.1%) were infiltrating adenocarcinoma with mucinous differentiation 14 (9.5%) were mucinous carcinoma, 3 (2%) were signet ring cell carcinoma, 1 (0.7%) was adenocarcinoma with neuroendocrine differentiation.
Microscopic appearance of well, moderate and poorly differentiated adenocarcinoma of colon (left to right), (H&E 40X).

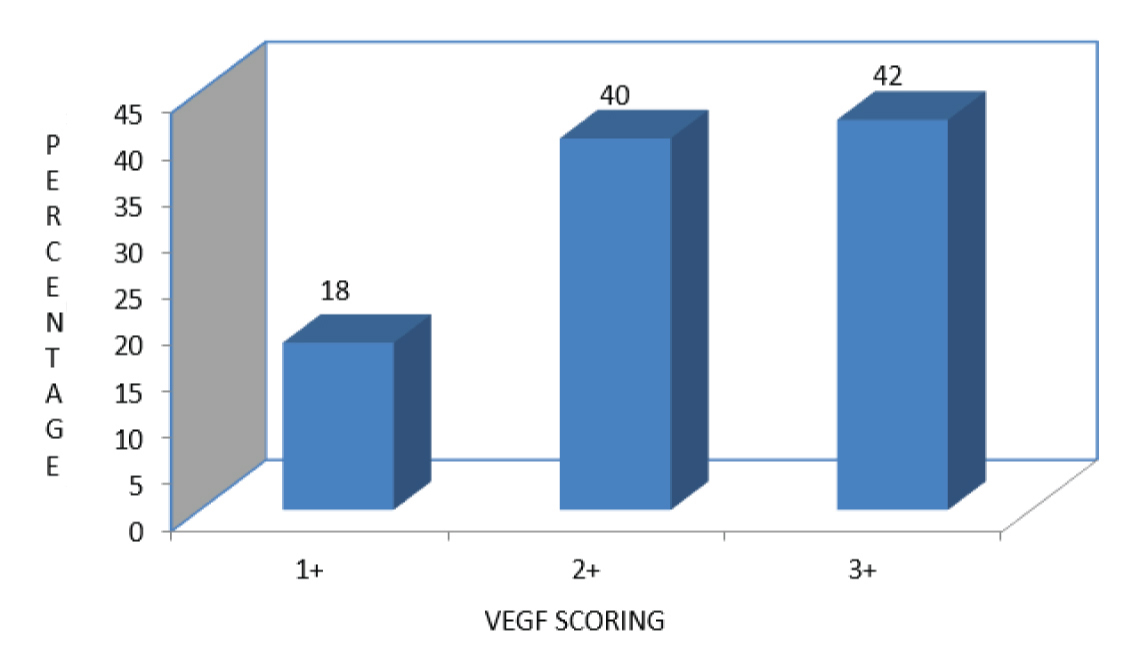
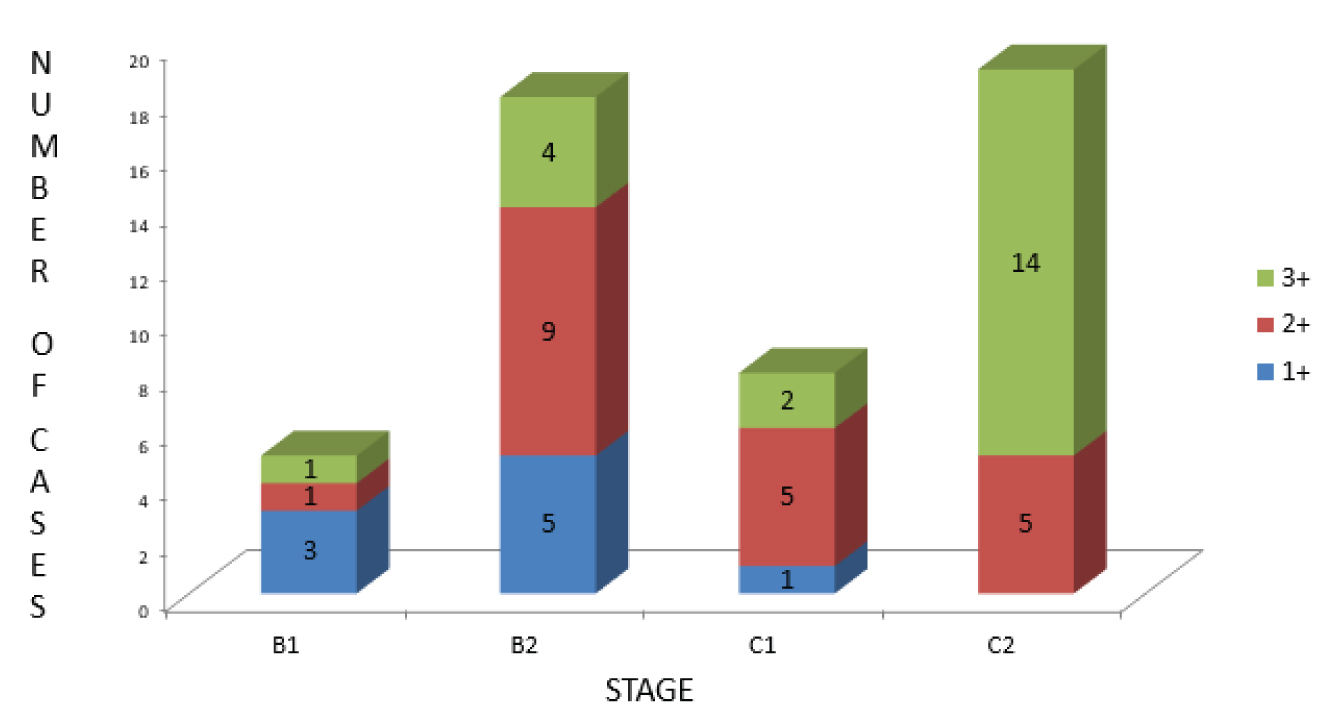
The detailed results of clinicopathological variables assessed in this study have been presented in [Table/Fig-4]. The percentage and scoring of VEGF positivity in the 50 random cases is given in [Table/Fig-5]. A total of 21 cases (42%) had strong cytoplasmic staining for VEGF and 15 cases (30%) and 14 cases (28%) showed moderate and weak cytoplasmic VEGF positivity respectively. The correlation between VEGF expression and parameters like lymphovascular invasion and lymph node involvement is given in [Table/Fig-6]. The correlation between tumour stage and levels of VEGF expression is given in [Table/Fig-7], p-value for correlation between VEGF expression and clinicopathological variables are given in [Table/Fig-8].
Details of clinicopathological variables assessed in this study.
| Variables | Frequency | Percent |
|---|
| Grade of differentiation | Well | 37 | 25.2 |
| Moderate | 95 | 64.6 |
| Poor | 15 | 10.2 |
| Stage of the tumour (Astler-Coller stage) | B1 | 36 | 24.5 |
| B2 | 44 | 29.9 |
| C1 | 14 | 9.5 |
| C2 | 53 | 36.1 |
| Lymphatic invasion | Present | 74 | 50.3 |
| Absent | 73 | 49.7 |
| Vascular invasion | Present | 67 | 45.6 |
| Absent | 80 | 54.4 |
| Lymph node involvement | Present | 66 | 44.9 |
| Absent | 81 | 55.1 |
| Lymphocytic response | Present | 76 | 51.7 |
| Absent | 71 | 48.3 |
| Resected margins | Free | 143 | 97.3 |
| Involved | 4 | 2.7 |
The percentage and scoring of VEGF positivity in the 50 random cases.
The correlation between VEGF expression and parameters like lymphovascular invasion and lymph node involvement.
| Variables | VEGF positivity |
|---|
| 1+ | 2+ | 3+ |
|---|
| Lymphatic invasion | Present | 2 (22.2%) | 14 (70%) | 19 (90.5%) |
| Absent | 7 (77.8%) | 6 (30%) | 2 (9.5%) |
| Vascular invasion | Present | 3 (33%) | 15 (75%) | 17 (81%) |
| Absent | 6 (67%) | 5 (25%) | 4 (19%) |
| Lymph node involvement | Present | 1 (11.1%) | 10 (50%) | 16 (76.2%) |
| Absent | 8 (88.9%) | 10 (50%) | 5 (23.8%) |
The correlation between tumour stage and levels of VEGF expression.
Shows p-values for correlation between VEGF expression and clinicopathological variables.
| Variables | Pearson Chi-square value | p-value |
|---|
| Gender | 0.740 | 0.691 |
| Location of the tumour | 3.653 | 0.161 |
| Size of the tumour | 3.229 | 0.199 |
| Grade of the tumour | 4.467 | 0.107 |
| Lymphovascular invasion | 7.20 | 0.027 |
| Lymph node involvement | 10.956 | 0.004 |
| Stage of the tumour | 19.607 | <0.05 |
Discussion
Globally, colorectal cancer poses a major public health problem. Survival is mainly dependent on the stage of the disease at diagnosis; with a better 5-year survival for patients diagnosed at the localised stage [9]. Many biological markers are being studied, in order to explore their prognostic significance. The history of targeted therapy dates back to 1971, when Folkman hypothesised that administration of an agent that prevents angiogenesis can have dramatic effect on cancer treatment [10]. In colon cancer, monoclonal antibodies against VEGF and Epidermal Growth Factor Receptor (EGFR) have been used for targeted therapies [11]. Cancers expressing VEGF can grow and metastasise. VEGF acts by binding to tyrosine kinase receptors on the cell surface. They are activated by transphosphorylation. The hypoxic cells produce Hypoxia Inducible Factor (HIF) which stimulates the release of VEGF [12].
VEGF receptors are classified into VEGF-R1, VEGF-R2, VEGF-R3. VEGF-R2 is located in vascular endothelial cells and VEGF-R3 on lymphatic endothelial cells and stimulate angiogenesis and lymphangiogenesis respectively [13]. Circulating VEGF binds to VEGF receptor on endothelial cells triggering tyrosine kinase pathway leading to angiogenesis [13]. Thus, assessing the levels of expression of VEGF in colorectal cancer can predict the extent of the ability of the tumour to metastasise.
Many studies have been conducted based on the expression of VEGF in colorectal cancer [14,15]. Troisi RJ et al., Burshey RP et al., and Chu KC et al., conducted studies in 223, 168 and 108 colorectal carcinoma patients and found that the mean age at diagnosis was 60, 72 and 58 years respectively [16-18]. The median age at diagnosis of colorectal carcinoma in this study was 60 years which was in concurrence with the study done by Troisi RJ et al., [16].
Side of the primary tumour was in concurrence with a study done by El-Gendi S and Al-Gendi A, in which left sided tumours constituted about 75% and 25% were right sided tumours [19]. In the study conducted by Kazem A et al., in 323 colorectal cancer patients, left sided tumours were 56.7% and 43.3% tumours were right-sided [20]. Also, the most common histological type was infiltrating adenocarcinoma (86.7%) followed by mucinous carcinoma (10%). In our study, it was infiltrating adenocarcinoma (83.7%) followed by mucinous adenocarcinoma (9.5 %) and adenocarcinoma with mucinous differentiation (4.1%).
On comparison with the clinicopathological parameters, there was a statistically significant association among the levels of VEGF expression by immunohistochemistry and stage of the tumour, the presence of lymphovascular invasion and lymph node metastasis.
In a study conducted by Bendarafa R et al., in 360 colorectal cancer patients, cytoplasmic VEGF expression in the tumour cells was assessed by automated immunohistochemistry [21]. Significant statistical association was found between VEGF expression and factors like, location of the tumour (left sided tumours expressed VEGF more than the right sided tumours), stage of the disease and 10-year disease specific survival. Thus, assessing VEGF expression in colorectal carcinoma helped in selecting patients who are likely to benefit from neoadjuvant chemotherapy.
Zafirellis K et al., did a study in 117 colorectal cancer patients and assessed the cytoplasmic VEGF expression in the tumour cells using semi-automated computerised image analysis [22]. Statistically significant association was found between presence of lymph node metastasis and stage of the disease. High levels of VEGF staining was seen to correlate with poor disease-specific survival (p<0.0001). Thus, VEGF expression in colorectal cancer seems to be an independent prognostic factor and helps to identify patients with unfavourable clinical outcome.
In studies, conducted by Takahashi Y et al., Kang SM et al., and Takahashi Y et al., among 52, 156 and 27 colorectal carcinoma patients respectively, significant statistical association was found between presence of lymphovascular invasion and lymph node involvement [23-25]. In studies conducted by Martin SF et al., Yeh CY et al., Guetz GD et al., and Ogata Y et al., in 672, 58, 317 and 92 colorectal carcinoma patients respectively, there is significant association between VEGF expression and lymph node involvement [26-29].
Also in studies conducted by Bendarafa R et al., Zafirellis K et al., Okita NT et al., Ochs AM et al., in 360, 117, 91, and 109 colorectal carcinoma patients, significant statistical association was found between VEGF expression and stage of the tumour [21,22,30,31].
Limitation
In various studies conducted among colorectal cancer patients, the higher levels of VEGF expression correlated with disease specific survival of the patients. But in this study, the patients were not followed up.
Conclusion
To conclude, this study assessed the expression of VEGF in colorectal cancer by immunohistochemistry which was found to have a statistically significant association with clinicopathologic characteristics that contribute to progression, invasion and metastasis, thereby leading to poor prognosis. Thus, assessing VEGF expression in colorectal cancer provides valuable prognostic information and helps to identify patients with an unfavourable clinical outcome.
Also, this study throws light on the need for targeted therapy with anti-VEGF drugs to minimise pathological vasculogenesis in the tumour, occurrence of regional and distant metastasis, thereby improving patient survival.