Case Report
A 19-year-old American Indian male patient presented to our facility with a two week history of right upper quadrant abdominal pain. The pain was sharp and radiated to his back. He reported one episode of bilious vomiting but denied fever, cough, dyspnoea, dysuria, diarrhoea, constipation, haematemesis, melena haematochezia and haematuria. Review of systems was significant for polydipsia and polyuria. His medical history was significant for poorly controlled type 1 diabetes with recurrent Diabetic Ketoacidosis (DKA) related admissions. His home diabetic regimen included levemir 20 units twice daily and a sliding-scale novolog with meals. He was an active polysubstance abuser (nicotine, alcohol and methamphetamine) and had compliance issues with his home medications.
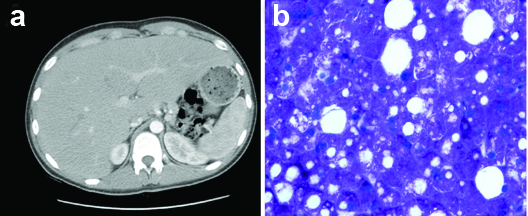
His blood pressure was 124/90 mmHg, heart rate 128 beats/minute, respiratory rate 16 breaths/minute, SpO2 100% on room air, temperature 98oF and Body Mass Index (BMI) 20.6 kg/m2. Physical examination revealed a soft, flat abdomen with mild tenderness over the right upper quadrant. The liver was firm, smooth and tender and approximately three finger breadths below the costal margin in the right midclavicular line. The remainder of the physical examination was unremarkable. Laboratory analysis revealed elevated serum glucose of 296 mg/dL, increased anion gap of 35, positive 2+ urine ketones, serum bicarbonate of 14 mmol/L, and serum lactic acid of 6.5 mmol/L with venous pH of 7.39. Liver panel showed elevated Aspartate Aminotransferase (AST) 165 U/L, Alanine Aminotransferase (ALT) 189 U/L, Alkaline Phosphatase (ALP) 186 U/L and a normal total bilirubin of 0.2 mg/dL. Both HbA1c and serum triglycerides levels were elevated at 11% and 549 mg/dL respectively. Complete blood count and coagulation profiles were within the normal reference range. Computed Tomography (CT) scan of the abdomen showed hepatomegaly of 30 cm without any focal lesions or intrahepatic ductal dilation [Table/Fig-1a]. Ultrasonography of liver revealed hepatomegaly with increased echogenicity consistent with an infiltrative process. Ultrasonography of the gall bladder and biliary system was otherwise unremarkable. The patient was admitted to the Intensive Care Unit (ICU) and treated for DKA. He subsequently underwent a liver biopsy and the histopathologic findings were consistent with GH [Table/Fig-1b]. After resolution of DKA, he was restarted on his home insulin regimen consisting of levemir 20 units BID and insulin coverage based on a sliding scale glucose level. His glucose levels were well controlled on this regimen. Since, his blood sugar level was reasonably well-controlled on the insulin regimen that was supposed to be at home, we concluded that medication non compliance was the reason for his poorly controlled diabetes and associated hepatopathy. Extensive diabetic education was provided to him during his short hospital stay. The plan was to monitor his diabetes and the underlying liver disease as an outpatient. However, he did not keep his outpatient follow up appointments and was subsequently lost to follow up.
a) CT scan of the abdomen showing hepatomegaly without any focal liver lesions; b) Hepatocytes with glycogen overload seen on periodic acid-schiff (PAS) staining.
Discussion
Glycogenic hepatopathy was first described by Mauriac in 1930 in an uncontrolled type 1 diabetic child as a component of Mauriac syndrome (growth failure, hyperlipidaemia, delayed puberty, hepatomegaly and cushingoid appearance) and has been reported in handful of case reports [1,2]. However, GH has also been described in patients without the extra-hepatic manifestations of Mauriac syndrome [3]. The prevalence of GH in patients with hepatomegaly and transaminitis is close to 80% among uncontrolled diabetics [4]. Yet this entity remains largely underdiagnosed and therefore, under reported consequently, the overall prevalence of GH is not known exactly.
Non Alcoholic Fatty Liver Disease (NAFLD) commonly presents with hepatomegaly and transaminitis, as a result it is commonly assumed to be the diagnosis. Non Alcoholic Steatohepatitis (NASH), GH and other causes of acute or chronic hepatitis must be considered in the differentials when these patients present with transaminitis. NAFLD is the most common chronic liver disease worldwide with reported global prevalence of 25% [5]. Discriminating between these diseases is important because the prognosis are different. Glycogenic hepatopathy is completely reversible with institution of glucose control; in contrast NAFLD may lead to fibrosis and cirrhosis. Ultrasonography, CT and Magnetic Resonance Imaging (MRI) cannot differentiate between NAFLD and GH and a definitive diagnosis of GH can only be made by liver biopsy [6].
NAFLD is the most common liver disorder worldwide especially in the western industrialised nations. NAFLD is characterised by fatty infiltration and elevated transaminases; whereas, NASH also has signs of inflammation. There is no good correlation between glycaemic control and the degree of steatosis [7]. Furthermore, a negative ultrasound does not exclude steatosis; thus, necessitating a biopsy to diagnose or assess the degree of fibrosis [8,9].
Patients with NAFLD, acute hepatitis and GH may present with fatigue, abdominal pain, acute transaminitis and hepatomegaly. NASH and hepatitis may progress to cirrhosis or hepatocellular carcinoma; in contrast, GH is caused by poor glucose control and has been shown to be completely reversible with stabilisation of glycaemic control [5,10]. While, GH has also been reported in diabetics with poorly controlled type 2 insulin-dependent diabetes, it has also been described in various circumstances involving poor glycaemic control: a type 2 diabetic patient who attempted suicide by injecting 180 units of insulin glargine, in non diabetics including a toddler with dumping syndrome associated with gastrostomy feeding and three children treated with high-dose glucocorticoid therapy [11-13].
Hyperglycaemia causes inhibition of the glycogen phosphorylase flux; whereas, hyperinsulinemia cause stimulation of the glycogen synthase flux. Both these steps occurring simultaneously cause a major suppression in the hepatic glycogen cycling and an increase in the hepatic glycogen synthesis [14]. This mechanism is thought to underlie the pathogenesis of GH in young diabetics with poorly controlled diabetes. Recently, a mutation in the catalytic subunit of liver glycogen phosphorylase kinase has been attributed to the development of GH [15]. Strict glycaemic control must be obtained in order to treat GH and reverse the hepatomegaly and transaminitis [10]. The converse has also been reported three patients who were diagnosed with GH demonstrated complete reversal of symptoms without intense glucose control, their HbA1c levels being 11%, 13% and 15%, respectively [16].
Review of literature indicated that there were 124 reported cases of GH, the details of which are summarised in [Table/Fig-2,3,4]. The median age was 19±10.6 years (range 2-70 years). Most of the patients were uncontrolled type 1 diabetics (93.7%), only two were type 2 diabetics (1.6%) and six (4.7%) were not diabetic. Only 11 (8.7%) presented with acute DKA. The most common physical finding was hepatomegaly with 63 patients (49.6%) having a palpable hepatomegaly at presentation. Forty patients (31.5%) had severely elevated AST and ALT (defined as >3x ULN). The median HbA1c was 11.7%±2.7 (n=47, range 6.2-22.2%), with the lowest HbA1c finding occurring in a patient with new-onset fulminant type 1 diabetes mellitus who was found in cardiac arrest. The median AST, ALT, ALP and Gamma-glutamyltransferase (GGT) values were 363±1451 U/L (n=55, range 9-6720); 346±505 U/L (n=59, range 14-2549); 199±240 U/L (n=30, range 79-933) and 187±319 U/L (n=25, range 15-1467), respectively. Total bilirubin was found to be elevated in eight patients (6.3%) and eight patients had lactic acidosis. Of the reported cases, 104 patients (81.9%) underwent liver biopsy, all were positive for GH; however, 21 also had steatosis and another 15 also had fibrosis.
Cases of glycogenic hepatopathy, presentation and time to normalisation of liver size and liver function tests (LFT).
| Age/Sex (years) | Presentation | Time to normalisation | HbA1c | Reference |
|---|
| 21 M | New-onset fulminant T1DM with DKA, HM | Improvement at discharge | 6.2% | [6] |
| 20 F | T1DM, HM, transaminitis | Improvement at five-month follow up | 13.3% | [10] |
| 30 F | T2DM, HM, transaminitis, dietary indiscretion | Resolution at one month follow up | | [11] |
| 02 M | Dumping syndrome, no steroids or DM | n/a | | [12] |
| 10 F | T1DM, RUQ pain, HM, transaminitis | Persistent HM at two months | | [17] |
| 15 M | T1DM, DKA, HM, transaminitis | Persistent HM at five years | 9.6% | [18] |
| 18 F | T1DM, DKA, lactic acidosis, HM | Persistent lactic acidosis at six months | 11.4% | [19] |
| 21 F | T1DM, tender HM, transaminitis | Seven weeks | 8.1% | [20] |
| 19 F | T1DM, HM, DKA | Resolution 10 weeks after CSII | 14.6% | [21] |
| 29 F | T1DM, HM, anorexia nervosa | n/a | | [22] |
| 41 M | T2DM, HM, insulin overdose (SI), hypoglycemia | Began to resolve in eight days | 10.6% | [23] |
| 22 F | AN, transaminitis | n/a | | [24] |
| 26 F | AN, abdominal pain, transaminitis | Persistently transaminitis | | [25] |
| 22 M | T1DM, HM, transaminitis, anorexia | Improved with better glycaemic control | 11.3% | [26] |
| 18 F | T1DM, DKA (multiple episodes of DKA) | 10 days | 8.2% | [27] |
| 13 M | T1DM, DKA, ischemic hepatopathy | 16 days | 10.4% | [28] |
| 19 F | T1DM, transaminitis | Resolution at A1C of 9.1% | 10.1% | [29] |
| 17 F | T1DM, CD, HM, transaminitis | Resolved at one year follow up | 12.0% | [30] |
| 22 M | T1DM, AAT deficiency (ZZ), HM, transaminitis | Resolved with better glycaemic control | 14.6% | [31] |
| 16 M | T1DM, over insulinisation with dietary indiscretion, HM | Four weeks | 11.1% | [32] |
| 19 F | T1DM, occurred four weeks after treatment for DKA, HM | | | [33] |
| 21 F | T1DM, CD, HM, lactic acidosis, transaminitis | One week | 13.3% | [34] |
| 18 M | T1DM, abdominal pain, HM, lactic acidosis | Resolved at three-month follow up | 11.0% | [35] |
| 33 F | T1DM, transaminitis, diarrhoea | Normal at one year follow up | 13.7% | [36] |
| 8 M | T1DM, painful HM, transaminitis | Days | 14.0% | [37] |
| 27 M | T1DM, RUQ pain, HM, transaminitis | Resolution at three months | 15.0% | [38] |
| 19 M | T1DM, HM, transaminitis | Began to resolve in three weeks | | [39] |
| 13 M | T1DM, HM, transaminitis | | | [40] |
| 13 F | T1DM, HM, transaminitis | Began to resolve in four weeks | 10.7% | [41] |
| 12 F | Labile T1DM, HM, transaminitis | Began three weeks after CSII | 8.4% | [42] |
| 14 F | T1DM, RUQ pain, transaminitis | | 14.3% | [43] |
| 13 M | T1DM, HM, DKA | Improved at three months | | [44] |
| 13 M | T1DM, ketosis, HM | | | [45] |
| 19 M | T1DM, RUQ pain, HM, transaminitis, DKA, lactic acidosis | | 11% | Current report |
T1DM: type 1 diabetes mellitus, T2DM: type 2 diabetes mellitus, HM: hepatomegaly, DKA: diabetic ketoacidosis, CD: celiac disease, AN: anorexia nervosa, AAT: alpha-1-antitrypsin deficiency, SI: suicidal ideation (attempt), CSII: continuous subcutaneous insulin infusion.
Summary of glycogenic hepatopathy case reports involving multiple patients.
| Number | Summary | Resolution | Reference |
|---|
| 2 | Newly diagnosed males T1DM, HM (both) | Weeks | [3] |
| 3 | Ages 8-12, two females, HM (all), high dose steroids, no DM | n/a | [13] |
| 3 | Ages 20-26, three females, T1DM, GH with persistent transaminitis, mean A1C 13.4% (±0.47) | Four to eight weeks, still poor DM control | [16] |
| 14 | Ages 8-25, nine males, T1DM, abdominal pain, transaminitis, HM | Three months (three patients) | [46] |
| 2 | 18/F and 21/M with labile T1DM, HM (both) | Cure with pancreas transplant | [47] |
| 11 | Ages 13-70, median age 23, seven females T1DM (all), HM (nine), transaminitis (six) | Two weeks | [48] |
| 3 | Adolescents, two males, T1DM, HM, transaminitis | Resolved with better glycaemic control | [49] |
| 3 | Ages 13-14, two females, T1DM, HM (all), mean A1C 12.5% (±1.35) | Two weeks | [50] |
| 4 | Ages 17-20, median age 19, three females, T1DM, lactic acidosis (all), HM (all), mean A1C 12.5% (±2.14) | Normalized with glucose control (case four) | [51] |
| 4 | Ages 21-29, median age 21.5, three females, T1DM, DKA (three), HM (all), mean A1C 11.4% (±2.95) | Resolved with improved glycaemic control | [52] |
| 4 | Ages 19-29, median age 22.5, three females, T1DM, HM, transaminitis, mean A1C 13.2% (±2.46) | LFTs improved in one month | [53] |
| 4 | Ages 12-18, median age 13, three females, T1DM with multiple episodes of DKA, mean A1C 11.9% (±1.80) | Four weeks | [54] |
Summary of GH case reports.
| Summary of GH Case Reports |
|---|
| Total number of patients | N=124 |
|---|
| Diabetes mellitus | 118 (95.1%) |
| Type 1 | 116 |
| Type 2 | 2 |
| Non-diabetic | 6 (4.9%) |
| Diabetic ketoacidosis (at presentation) | 11 (8.8%) |
| Hepatomegaly (physical examination) | 63 (50.8%) |
| Biopsy | 104 |
| Glycogenic hepatopathy | 104 (100%) |
| Steatosis | 21 (20.2%) |
| Fibrosis | 15 (14.4%) |
| Inflammation | 1 (1%) |
| Hepatitis | 1 (1%) |
| Elevated liver enzymes (peak) |
| AST (40 U/L=ULN) |
| Mild (<3x ULN) | 10 (8%) |
| Severe (>3x ULN) | 40 (32.2%) |
| ALT (40 U/L=ULN) |
| Mild (<3x ULN) | 14 (11.3%) |
| Severe (>3x ULN) | 40 (32.2%) |
| ALP (130 U/L=ULN) |
| Mild (<3x ULN) | 23 (18.5%) |
| Severe (>3x ULN) | 9 (7.2%) |
| GGT (45 U/L=ULN) |
| Mild (<3x ULN) | 9 (7.2%) |
| Severe (>3x ULN) | 14 (11.3%) |
| Total bilirubin (>1.0 mg/dL) | 8 (6.4%) |
| Elevated lactic acid (>2.2 mmol/L) | 8 (6.4%) |
[Table/Fig-5] summarises the clinical characteristics of two cohort studies. In the large cohort, which assessed the prevalence of liver dysfunction in 692 children with type 1 diabetes mellitus, 60 (8.7%) had one or more abnormalities hepatomegaly, increased ALT, positive anti-HCV antibody and abnormal USG. These abnormalities were reported to resolve in 37 of the 60 with improved glycaemic control. Of these, we only included the three biopsy confirmed cases; although, more are suspected. The second cohort examined the liver of 31 children with Mauriac syndrome and GH, of which 19 had biopsies performed. All the biopsies were positive for GH.
List of cohort studies reviewed involving glycogenic hepatopathy.
| Summary | Reference |
|---|
| N=31 (16 males) with GH. Median age 15.1 years. Liver biopsy was done showing GH. 17 children improved on follow up. | [2] |
| N=692 (333 males) with T1DM. Mean age 9.7 years. 60 (8.7%) of the 692 studied had either hepatomegaly, increased ALT, positive HCV antibody or abnormal liver US. Biopsy showed GH in three cases. | [55] |
Our case is the only documented case of active methamphetamine use which has also been shown to cause GH; although, the mechanism is not completely understood. Our patient also presented with lactic acidosis. Generally, resolution of hepatomegaly and transaminitis occurs quickly, most often between two to four weeks. Strict glycaemic control must be obtained in order treat GH and reverse the hepatomegaly and transaminitis. However, two patients with persistent hepatomegaly one at two months and the other at five years have been described [17,18]. One of the cases also had persistent lactic acidosis [19]. Until more is known about this entity; we would emphasise good glycaemic control for patients with GH as it would also reduce other complications related to diabetes.
Conclusion
Non-alcoholic fatty liver disease, NASH, chronic hepatitis and GH may all present with right upper quadrant pain, hepatomegaly and transaminitis. Liver biopsy is the gold standard for diagnosis as imaging by ultrasound cannot reliably distinguish between them. NAFLD, NASH and chronic hepatitis can lead to fibrosis and eventually progress to cirrhosis and hepatocellular carcinoma; whereas, GH has a good prognosis and completely resolves on institution of adequate glycaemic control.
T1DM: type 1 diabetes mellitus, T2DM: type 2 diabetes mellitus, HM: hepatomegaly, DKA: diabetic ketoacidosis, CD: celiac disease, AN: anorexia nervosa, AAT: alpha-1-antitrypsin deficiency, SI: suicidal ideation (attempt), CSII: continuous subcutaneous insulin infusion.
[1]. Kim MS, Quintos JB, Mauriac syndrome: growth failure and type 1 diabetes mellitusPediat Endocrinology Rev 2008 4:989-93. [Google Scholar]
[2]. Fitzpatrick E, Cotoi C, Quaglia A, Sakellariou S, Ford-Adams ME, Hadzic N, Hepatopathy of Mauriac syndrome: a retrospective review from a tertiary liver centreArchives of Disease in Childhood 2014 99(4):354-57.10.1136/archdischild-2013-30442624412980 [Google Scholar] [CrossRef] [PubMed]
[3]. Carcione L, Lombardo F, Messina MF, Rosano M, De Luca F, Liver glycogenosis as early manifestation in type 1 diabetes mellitusDiabetes Nutr Metab 2003 16(3):182-84. [Google Scholar]
[4]. Stone BG, Van Thiel DH, Diabetes mellitus and the liverSemin Liver Dis 1985 5(1):08-28.10.1055/s-2008-10417543885402 [Google Scholar] [CrossRef] [PubMed]
[5]. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M, Global epidemiology of nonalcoholic fatty liver disease meta-analytic assessment of prevalence, incidence, and outcomesHepatology 2016 64(1):73-84.10.1002/hep.2843126707365 [Google Scholar] [CrossRef] [PubMed]
[6]. Murata F, Horie I, Ando T, Isomoto E, Hayashi H, Akazawa S, A case of glycogenic hepatopathy developed in a patient with new-onset fulminant type 1 diabetes: the role of image modalities in diagnosing hepatic glycogen deposition including gradient-dual-echo MRIEndocr J 2012 59(8):669-76.10.1507/endocrj.EJ12-008122673296 [Google Scholar] [CrossRef] [PubMed]
[7]. Silverman JF, O’Brien KF, Long S, Leggett N, Khazanie PG, Pories WJ, Liver pathology in morbidly obese patients with and without diabetesAm J Gastroenterol 1990 85(10):1349-55. [Google Scholar]
[8]. Scatarige JC, Scott WW, Donovan PJ, Siegelman SS, Sanders RC, Fatty infiltration of the liver: ultrasonographic and computed tomographic correlationJ Ultrasound Med 1984 3(1):09-14.10.7863/jum.1984.3.1.96694259 [Google Scholar] [CrossRef] [PubMed]
[9]. Leevy CM, Fatty liver: a study of 270 patients with biopsy proven fatty liver and review of the literatureMedicine (Baltimore) 1962 41:249-76.10.1097/00005792-196209000-0000314463641 [Google Scholar] [CrossRef] [PubMed]
[10]. Hudacko RM, Manoukian AV, Schneider SH, Fyfe B, Clinical resolution of glycogenic hepatopathy following improved glycemic controlJ Diabetes Complications 2008 22(5):329-30.10.1016/j.jdiacomp.2007.11.00418413180 [Google Scholar] [CrossRef] [PubMed]
[11]. Nakamuta M, Ohashi M, Goto K, Tanabe Y, Hiroshige K, Nawata H, Diabetes mellitus-associated glycogen storage hepatomegaly: report of a case and review of the Japanese literatureFukuoka Igaku Zasshi 1993 84(7):354-58. [Google Scholar]
[12]. Resnick JM, Zador I, Fish DL, Dumping syndrome: a cause of acquired glycogenic hepatopathyPediatr Dev Pathol 2011 14(4):318-21.10.2350/10-07-0876-CR.121338321 [Google Scholar] [CrossRef] [PubMed]
[13]. Iancu TC, Shiloh H, Dembo L, Hepatomegaly following short-term high-dose steroid therapyJ Pediatr Gastroenterol Nutr 1986 5(1):41-46.10.1097/00005176-198601000-000083944744 [Google Scholar] [CrossRef] [PubMed]
[14]. Ferrer JC, Favre C, Gomis RR, Fernandez-Novell JM, Garcia-Rocha M, de la Iglesia N, Control of glycogen depositionFEBS Lett 2003 546(1):127-32.10.1016/S0014-5793(03)00565-9 [Google Scholar] [CrossRef]
[15]. MacDonald MJ, Hasan NM, Ansari IU, Longacre MJ, Kendrick MA, Stoker SW, Discovery of a Genetic Metabolic Cause for Mauriac Syndrome in Type 1 DiabetesDiabetes 2016 65(7):2051-59.10.2337/db16-009927207549 [Google Scholar] [CrossRef] [PubMed]
[16]. Cha JH, Ra SH, Park YM, Ji YK, Lee JH, Park SY, Three cases of glycogenic hepatopathy mimicking acute and relapsing hepatitis in type I diabetes mellitusClinical and Molecular Hepatology 2013 19(4):421-25.10.3350/cmh.2013.19.4.42124459648 [Google Scholar] [CrossRef] [PubMed]
[17]. Jin HY, Kang D-Y, Choi JH, Hepatic glycogenosis in a patient with poorly controlled type 1 diabetes mellitusKorean J Pediatr 2009 52(11):1279-82.10.3345/kjp.2009.52.11.1279 [Google Scholar] [CrossRef]
[18]. Kant R, Loper HB, Verma V, Malek R, Drachenberg CB, Munir KM, Glycogenic hepatopathy with persistent hepatomegaly in a patient with uncontrolled type 1 diabetesJournal of Endocrinology and Metabolism 2015 5(1-2):189-91.10.14740/jem276w [Google Scholar] [CrossRef]
[19]. Deemer KS, Alvarez GF, A rare case of persistent lactic acidosis in the icu: glycogenic hepatopathy and mauriac syndromeCase Reports in Critical Care 2016 2016:6072909 [Google Scholar]
[20]. Torres M, Lopez D, Liver glycogen storage associated with uncontrolled type 1 diabetes mellitusJ Hepatol 2001 35(4):53810.1016/S0168-8278(01)00132-5 [Google Scholar] [CrossRef]
[21]. Imtiaz KE, Healy C, Sharif S, Drake I, Awan F, Riley J, Glycogenic hepatopathy in type 1 diabetes: an underrecognized conditionDiabetes Care 2013 36(1):e6-e7.10.2337/dc12-113423264308 [Google Scholar] [CrossRef] [PubMed]
[22]. Van den Brand M, Elving LD, Drenth JP, van Krieken JH, Glycogenic hepatopathy: a rare cause of elevated serum transaminases in diabetes mellitusNeth J Med 2009 67(11):394-96. [Google Scholar]
[23]. Tsujimoto T, Takano M, Nishiofuku M, Yoshiji H, Matsumura Y, Kuriyama S, Rapid onset of glycogen storage hepatomegaly in a type-2 diabetic patient after a massive dose of long-acting insulin and large doses of glucoseIntern Med 2006 45(7):469-73.10.2169/internalmedicine.45.154816679704 [Google Scholar] [CrossRef] [PubMed]
[24]. Komuta M, Harada M, Ueno T, Uchimura Y, Inada C, Mitsuyama K, Unusual accumulation of glycogen in liver parenchymal cells in a patient with anorexia nervosaIntern Med 1998 37(8):678-82.10.2169/internalmedicine.37.6789745854 [Google Scholar] [CrossRef] [PubMed]
[25]. Kransdorf LN, Millstine D, Smith ML, Aqel BA, Hepatic glycogen deposition in a patient with anorexia nervosa and persistently abnormal transaminase levelsClinics and Research in Hepatology and Gastroenterology 2016 40(2):e15-e18.10.1016/j.clinre.2015.05.00126066296 [Google Scholar] [CrossRef] [PubMed]
[26]. Park J, Song D, Park JS, Nam JY, Kim CS, Kim DM, A case of hepatomegaly due to diabetic glycogenosis reversed by glycemic controlJ Korean Soc Endocrinol 2004 19(2):223-28. [Google Scholar]
[27]. Lee SH, Kwon HS, Shin JA, Kim WC, Kim JH, Choi YH, A case of hepatic glycogenosis in a patient with uncontrolled type 1 diabetes mellitusJ Korean Diabetes Assoc 2006 30(1):82-86.10.4093/jkda.2006.30.1.82 [Google Scholar] [CrossRef]
[28]. Martin J, Tomlinson P, Hepatic complications in poorly controlled type 1 diabetes mellitus: a case reportThe New Zealand Medical Journal 2014 127(1392):95-97. [Google Scholar]
[29]. Bassett JT, Veerappan GR, Lee DH, Glycogenic hepatopathy: a rare cause of increased aminotransferase levels in a diabetic patientClin Gastroenterol Hepatol 2008 6(11):A2610.1016/j.cgh.2008.04.03418691943 [Google Scholar] [CrossRef] [PubMed]
[30]. Bua J, Marchetti F, Faleschini E, Ventura A, Bussani R, Hepatic glycogenosis in an adolescent with diabetesJ Pediatr 2010 157(6):104210.1016/j.jpeds.2010.06.01820638077 [Google Scholar] [CrossRef] [PubMed]
[31]. Xu J, Dhall D, Sundaram V, An enlarging liver in a young diabetic maleGastroenterology 2015 149(6):e8-e10.10.1053/j.gastro.2015.02.02426433109 [Google Scholar] [CrossRef] [PubMed]
[32]. Abaci A, Bekem O, Unuvar T, Ozer E, Bober E, Arslan N, Hepatic glycogenosis: a rare cause of hepatomegaly in Type 1 diabetes mellitusJournal of Diabetes and its Complications 2008 22(5):325-28.10.1016/j.jdiacomp.2007.11.00218413182 [Google Scholar] [CrossRef] [PubMed]
[33]. Tomihira M, Kawasaki E, Nakajima H, Imamura Y, Sato Y, Sata M, Intermittent and recurrent hepatomegaly due to glycogen storage in a patient with type 1 diabetes: genetic analysis of the liver glycogen phosphorylase gene (PYGL)Diabetes Res Clin Pract 2004 65(2):175-82.10.1016/j.diabres.2003.12.00415223230 [Google Scholar] [CrossRef] [PubMed]
[34]. Parmar N, Atiq M, Austin L, Miller RA, Smyrk T, Ahmed K, Glycogenic Hepatopathy: Thinking Outside the BoxCase reports in gastroenterology 2015 9(2):221-26.10.1159/00043704826269698 [Google Scholar] [CrossRef] [PubMed]
[35]. Saadi T, Glycogenic hepatopathy: a rare disease that can appear and resolve rapidly in parallel with glycemic controlIsr Med Assoc J 2012 14(4):269-70. [Google Scholar]
[36]. Saxena P, Turner I, McIndoe R, Education and Imaging. Hepatobiliary and pancreatic: Glycogenic hepatopathy: a reversible conditionJ Gastroenterol Hepatol 2010 25(3):64610.1111/j.1440-1746.2010.06178.x20370736 [Google Scholar] [CrossRef] [PubMed]
[37]. Lecomte M, Gottrand F, Stuckens C, Lecomte-Houcke M, Acute steatosis in an 8-year-old boy with insulin-dependent diabetes mellitusJournal of Pediatric Gastroenterology and Nutrition 1997 25(1):98-100.10.1097/00005176-199707000-000179226536 [Google Scholar] [CrossRef] [PubMed]
[38]. Sweetser S, Kraichely RE, The bright liver of glycogenic hepatopathyHepatology 2010 51(2):711-12.10.1002/hep.2340219957373 [Google Scholar] [CrossRef] [PubMed]
[39]. Atmaca M, Ucler R, Kartal M, Seven I, Alay M, Bayram I, Glycogenic Hepatopathy in Type 1 Diabetes MellitusCase reports in Hepatology 2015 2015:23614310.1155/2015/23614326347835 [Google Scholar] [CrossRef] [PubMed]
[40]. Aljabri KS, Bokhari SA, Fageeh SM, Alharbi AM, Abaza MA, Glycogen hepatopathy in a 13-year-old male with type 1 diabetesAnn Saudi Med 2011 31(4):424-27.10.4103/0256-4947.8180321727748 [Google Scholar] [CrossRef] [PubMed]
[41]. Jeong HR, Shim YS, Kim YB, Lee HS, Hwang JS, Glycogenic hepatopathy in a Korean girl with poorly controlled type 1 diabetes mellitusAnnals of Pediatric Endocrinology and Metabolism 2014 19(1):49-52.10.6065/apem.2014.19.1.4924926465 [Google Scholar] [CrossRef] [PubMed]
[42]. Chung IH, Jeong SJ, Cho YA, Kim GI, Yoo EG, Liver dysfunction due to hepatic glycogenosis in a girl with type 1 diabetesJ Korean Soc Pediatr Endocrinol 2009 14(2):174-78.10.3803/jkes.2009.24.3.174 [Google Scholar] [CrossRef]
[43]. Jung IA, Cho WK, Jeon YJ, Kim SH, Cho KS, Park SH, Hepatic glycogenosis in type 1 diabetes mellitus mimicking Mauriac syndromeKorean J Pediatr 2015 58(6):234-37.10.3345/kjp.2015.58.6.23426213553 [Google Scholar] [CrossRef] [PubMed]
[44]. Ruschhaupt DG, Rennert OM, Recurrent hepatomegaly and transient alteration of liver functions in an adolescent with diabetic acidosisClinical Pediatrics 1970 9(2):122-24.10.1177/0009922870009002234984319 [Google Scholar] [CrossRef] [PubMed]
[45]. Weitzman S, Lehman E, Lischinsky M, Transient hepatomegaly with glycogen deposition in transient juvenile diabetes with ketosisHarefuah 1974 86(11):556-57. [Google Scholar]
[46]. Torbenson M, Chen YY, Brunt E, Cummings OW, Gottfried M, Jakate S, Glycogenic hepatopathy: an underrecognized hepatic complication of diabetes mellitusAm J Surg Pathol 2006 30(4):508-13.10.1097/00000478-200604000-0001216625098 [Google Scholar] [CrossRef] [PubMed]
[47]. Fridell JA, Saxena R, Chalasani NP, Goggins WC, Powelson JA, Cummings OW, Complete reversal of glycogen hepatopathy with pancreas transplantation: two casesTransplantation 2007 83(1):84-86.10.1097/01.tp.0000239510.27872.0717220798 [Google Scholar] [CrossRef] [PubMed]
[48]. Chatila R, West AB, Hepatomegaly and abnormal liver tests due to glycogenosis in adults with diabetesMedicine (Baltimore) 1996 75(6):327-33.10.1097/00005792-199611000-000038982149 [Google Scholar] [CrossRef] [PubMed]
[49]. Munns CF, McCrossin RB, Thomsett MJ, Batch J, Hepatic glycogenosis: reversible hepatomegaly in type 1 diabetesJ Paediatr Child Health 2000 36(5):449-52.10.1046/j.1440-1754.2000.00547.x11036799 [Google Scholar] [CrossRef] [PubMed]
[50]. Flotats BM, Miserachs BM, Ricart CA, Clemente LM, Gussinyer CM, Yeste F, Hepatomegaly due to glycogen storage disease and type 1 diabetes mellitusAnales de Pediatria 2007 67(2):157-60.10.1016/S1695-4033(07)70577-5 [Google Scholar] [CrossRef]
[51]. Brouwers MC, Ham JC, Wisse E, Misra S, Landewe S, Rosenthal M, Elevated lactate levels in patients with poorly regulated type 1 diabetes and glycogenic hepatopathy: a new feature of Mauriac syndromeDiabetes Care 2015 38(2):e11-e12.10.2337/dc14-220525614691 [Google Scholar] [CrossRef] [PubMed]
[52]. Silva M, Marques M, Cardoso H, Rodrigues S, Andrade P, Peixoto A, Glycogenic hepatopathy in young adults: a case seriesRev Esp Enferm Dig 2016 108(10):673-76.10.17235/reed.2016.3934/2015 [Google Scholar] [CrossRef]
[53]. Ikarashi Y, Kogiso T, Hashimoto E, Yamamoto K, Kodama K, Taniai M, Four cases of type 1 diabetes mellitus showing sharp serum transaminase increases and hepatomegaly due to glycogenic hepatopathyHepatol Res 2017 47(3):e201-e209.10.1111/hepr.1271327027269 [Google Scholar] [CrossRef] [PubMed]
[54]. Vo HTD, Klein GW, Loizides A, Zhou P, Liu Q, Pan DH, Glycogen hepatopathy in children with poorly controlled type 1 diabetesInternational Journal of Case Reports and Images 2011 2(9):01-04.10.5348/ijcri-2011-09-51-CS-1 [Google Scholar] [CrossRef]
[55]. El-Karaksy HM, Anwar G, Esmat G, Mansour S, Sabry M, Helmy H, Prevalence of hepatic abnormalities in a cohort of Egyptian children with type 1 diabetes mellitusPediatric Diabetes 2010 11(7):462-70.10.1111/j.1399-5448.2009.00627.x20042012 [Google Scholar] [CrossRef] [PubMed]