One of the worldwide concerns is ID and IDA, which commonly occurs among children in the developing countries [1]. About 27% of children in Egypt have IDA [2].
Depletion of the body’s iron stores leads to ID which manifests early by elevated Red cell Distribution Width (RDW) and finally by occurrence of IDA which is diagnosed by low haemoglobin level (<11 gm/dL) and haematocrit value combined with low serum iron and low transferrin saturation [3].
Limited studies investigating the interaction and display of ID and thyroid hormones in humans are present [7,8]. We investigate the possible occurrence of thyroid dysfunction among children with isolated IDA and if the iron replacement therapy alone can reverse the associated thyroid function disturbances or additional therapies are required.
Materials and Methods
Study Population
This prospective study was carried out on 60 children in the age group from 2-16 years selected from the attendants to the outpatient paediatric clinics of Al-Azhar University, Assiut and Qena University Hospitals, upper Egypt, according to the inclusion criteria during the study period from 1st March 2016 to 28th February 2017. This was in addition to 60 apparently healthy age and sex matched children selected as control group. This study was conducted according to the guidelines laid down in the declaration of Helsinki [9], and all procedures involving human patients were approved by the Ethics Committee of both Faculty of Medicine, Al-Azhar University, Assiut and Faculty of Medicine, South Valley University, Qena, Egypt. Also, an informed consent was obtained from either parent of all included children.
Patients were selected according to the following inclusion criteria: any child aged 2-16 years old with clinical and laboratory diagnosis of isolated IDA, serum albumin, cholesterol and UIE, all to be within the normal range, his/her weight and height within normal range for the corresponding age and sex with normal Body Mass Index (BMI). Any child with the previous inclusion criteria, but with a history of receiving iron, other haematinics or multivitamins in the preceding three months, with acute or chronic illness, with chronic or recent acute blood loss, on hormonal therapy, with family history of hypothyroidism, with autoimmune thyroiditis, syndromic children, malnourished children, disturbed cholesterol or albumin serum levels, UIE less than 100 μg/L, C-reactive protein ≥0.6 mg/dL, were excluded from the study.
Laboratory Workup
A morning urine sample was obtained from each child and stored at -20°C until assay of urinary iodine by Sandell-Kolthoff reaction, using conventional chloric acid digestion method in a test tube; where, 250 μL from each urine sample or calibrators (25,50,100,200,300 and 400 μg/L iodine standard solutions) was added to 750 μL cholric acid solution, incubated for 60 minutes at 110°C, then 3.5 mL of arsenious acid was added to each test tube after cooling. Then, 350 μL of ceric ammonium sulfate solution added to each test tube and mixed properly. The absorbance at 405 nm was measured, using spectrophotometer (CHEM-7, Erba diagnostics Mannheim GmbH, Germany) [10]. Ten millilitres fasting venous blood samples were drawn from an antecubital vein of the included children, divided into two tubes, 2 mL on Ethylenediaminetetraacetic Acid (EDTA) tube for measurement of the complete blood count, using (Cell Dyn 1800-Abbott diagnostics, Germany) and 8 mL on serum separator gel tubes, were allowed to be clotted for 30 minutes then centrifuged for 15 minutes at 3000 rpm. Serum cholesterol and albumin of each sample were assessed by colorimetric methods [11-13], using Cobas C311 (Roche diagnostics, Germany) and the remaining amount was transferred and divided into aliquots using 1 mL cryotubes and stored at -20°C till time of biochemical analysis. All samples were measured in a single assay to avoid repeated freeze-thaw cycles. A commercially available ELISA kit was used for biochemical analysis of serum FT3 and FT4 (using solid phase competitive assay method), TSH and ferritin (using solid phase sandwich assay method) [14,15], all were supplied by Calbiotech Inc., 10461 Austin Dr, Spring Valley, CA, 91978. The catalog numbers were: F3106T for FT3, F4107T for FT4, S227T for TSH and R248T for ferritin. A commercially available colorimetric assay kit was used for measurement of serum iron and TIBC (supplied by Spectrum Diagnostics Co. Cairo, Egypt, Catalog No: 270001). TFS% was determined by dividing the serum iron concentration by the TIBC and multiplying by 100, unsaturated iron binding capacity (μg/dL), obtained by subtracting serum iron concentration from the TIBC [16,17].
Complete blood count, serum TSH, FT3, FT4, ferritin, iron, TIBC, TFS% and unsaturated iron binding capacity were measured in the studied groups at baseline, then haemoglobin level, serum ferritin, TSH, FT3, FT4 repeated among the studied patients after three months of oral iron supplementation therapy (6 mg/kg/day divided into two doses). Value of the thyroid profile of the included subjects to be judged as hypothyroidism should be less than the cut-off value of the standard of the normal reference range provided by the used kits, in children: in the present study, the normal reference ranges for TSH=0.35-4.94 μIU/mL, for FT3=1.71-4.8 pg/mL and for FT4= 0.7-1.48 ng/dL.
Anaemia can be considered in children when the levels of haemoglobin are less than 11 gm/dL and the severity of anaemia based on the haemoglobin levels to be considered as mild anaemia (10-10.9 gm/dL); moderate anaemia (7-9.9 gm/dL); severe anaemia (<7 gm/dL) [18]. Subclinical hypothyroidism considered when there is normal FT3 and FT4 levels with a slightly elevated TSH level [19], while primary hypothyroidism in which the defect in the thyroid gland itself, stated when there are decreased thyroid hormones concentration with subsequent elevated TSH level [20].
Statistical Analysis
Date entry and data analysis were done using the Statistical Package for the Social Sciences (SPSS) version 19.0. Data were presented as number, percentage, mean and standard deviation. Chi-square test was used to compare between qualitative variables. Mann-Whitney U test was used to compare between two quantitative variables in case of non parametric data. Wilcoxon signed rank test was done to compare quantitative variables before and after treatment. Spearman correlation was done to measure correlation between quantitative variables. MedCalc program was used to calculate sensitivity, specificity, positive and negative predictive values. The p-values were considered statistically significant when p<0.05.
Results
We enrolled 60 children with isolated IDA (38 males and 22 females), mean age 5.82 years (±3.05) and 60 controls (34 males and 26 females), mean age 6.28 years (±2.81). There were non significant differences in the mean levels of UIE, serum cholesterol and albumin among patients versus controls (p<0.05), which were all within the normal range to confirm the inclusion criteria as presented in [Table/Fig-1].
Comparison of the mean±SD of blood indices, iron study paramters, urinary iodine excretion, serum albumin and serum cholesterol among patients versus controls.
| Variables | Patients (n=60) | Control (n=60) | *p-value |
|---|
| mean±SD | mean±SD |
|---|
| Haemoglobin (gm/dL) | 9.18±1.39 | 11.80±0.80 | <0.001* |
| Haematocrit (%) | 27.36±3.55 | 34.36±3.20 | <0.001* |
| Red cell count (M/cmm) | 3.58±0.39 | 4.29±0.39 | <0.001* |
| Mean Corpuscular Volume (MCV) (fl) | 62.32±4.87 | 79.53±6.61 | <0.001* |
| Mean Corpuscular Haemoglobin (MCH) (pg) | 23.41±3.78 | 27.63±1.82 | <0.001* |
| Red cell Distribution Width (RDW) (%) | 16.76±2.66 | 14.60±1.53 | <0.001* |
| Ferritin (ng/mL) | 12.01±5.39 | 46.80±7.57 | <0.001* |
| Iron concentration (μg/dL) | 20.82±4.08 | 91.05±9.72 | <0.001* |
| Total Iron Binding Capacity (TIBC) (μg/dL) | 476.00±43.32 | 310.85±63.34 | <0.001* |
| Transferrin Saturation percent (TFS%) | 4.40±1.10 | 30.55±7.66 | <0.001* |
| Unsaturated Iron Binding Capacity (UIBC) (μg/dL) | 455.10±44.77 | 219.80±65.17 | <0.001* |
| Urinary iodine (μg/L) | 110.65±6.11 | 109.30±4.76 | 0.377 |
| Cholesterol (mg/dL) | 129.18±14.92 | 123.20±14.91 | 0.113 |
| Albumin (gm/dL) | 3.44±0.37 | 3.28±0.34 | 0.122 |
* Statistically significant difference (p<0.05)
Comparison of the mean±SD of blood indices and iron study parameters among patients versus controls is presented in [Table/Fig-1], which confirms the presence of microcytic hypochromic anaemia among the selected paediatric patients due to significant lower haemoglobin, haematocrit %, Red Blood Cells count (RBCs), Mean Corpuscular Volume (MCV), serum ferritin, iron and TFS% in the patient group versus the controls (p<0.001 for all).
Comparison of the mean±SD of TSH, FT4 and FT3 among patients versus controls is presented in [Table/Fig-2], which showed significantly higher serum TSH levels with significantly lower serum levels of FT3 and FT4 among patients versus controls (p<0.001 for all). The frequency of euthyroid status among the included patients (according to the supplied kit reference ranges for TSH, FT3 and FT4) was 48% (79% had mild anaemia and 21% had moderate anaemia) followed by subclinical hypothyroidism 28% (82% had moderate anaemia and 18% had severe anaemia), while, primary hypothyroidism has the lowest frequency 24% (all patients had severe anaemia).
Comparison of the mean±SD of TSH, FT4 and FT3 levels in patients in relation to the severity of anaemia was shown in [Table/Fig-3], with significant higher serum levels of TSH and significant lower FT3 in patients with severe IDA versus patients with moderate or mild IDA (p<0.01 for all).
Comparison of the mean±SD of thyroid stimulating hormone, FT4 and FT3 among patients versus controls.
| Thyroid profile parameters | Patients (n=60) | Controls (n=60) | *p-value |
|---|
| mean±SD | mean±SD |
|---|
| Thyroid stimulating hormone (μIU/mL) | 4.03±1.41 | 0.79±0.46 | <0.001* |
| Free T3 (pg/mL) | 1.03±0.12 | 4.08±0.70 | <0.001* |
| Free T4 (ng/dL) | 0.96±0.37 | 1.33±0.21 | <0.001* |
T3: Triiodothyronine, T4: Thyroxine
* Statistically significant difference (p<0.05)
Comparison of the mean±SD of thyroid stimulating hormone, FT4 and FT3 levels in patients in relation to the severity of anaemia.
| Thyroid profile parameters | Mild anaemia (n=23) | Moderate anaemia (n=20) | Severe anaemia (n=17) | **p-value |
|---|
| mean±SD | mean±SD | mean±SD | p1 | p2 | p3 |
|---|
| Thyroid stimulating hormone (μIU/mL) | 2.73±1.23 | 4.64±0.70 | 5.67±0.54 | <0.001* | <0.001* | <0.001* |
| Free T3 (pg/mL) | 1.05±0.08 | 1.04±0.15 | 0.93±0.03 | 0.829 | <0.001* | 0.006* |
| Free T4 (ng/dL) | 0.89±0.39 | 1.02±0.35 | 0.88±0.36 | 0.269 | 0.902 | 0.560 |
T3: Triiodothyronine, T4: Thyroxine
* Statistically significant difference (p<0.05)
** p1=mild anaemia versus moderate anaemia, p2=moderate anaemia versus severe anaemia, p3=mild anaemia versus severe anaemia
There was significant positive correlation between serum iron and FT3 (r=0.284, p<0.05) with significant negative correlations between TSH versus both serum iron (r=-0.635, p<0.001) and ferritin (r=-0.342, p<0.01) as presented in [Table/Fig-4]. Correlations between blood indices and thyroid profile among the included patients are presented in [Table/Fig-5], which revealed significant positive correlation between serum TSH levels and RDW (r=0.500, p<0.001) and between FT3 and RBCs (r=0.359, p<0.01) with significant negative correlations between serum TSH levels and MCV (r=-0.333, p<0.01), haemoglobin (r=-0.714, p<0.001), haematocrit % (r=-0.586, p<0.001) and RBCs (r=-0.298, p<0.05).
Correlations between the serum levels of ferritin, iron, TSH, FT3 and FT4.
| Variables | Ferritin (ng/mL) | Serum Iron Conc. (μg/dL) | Thyroid stimulating hormone (μIU/mL) | Free T3 (pg/mL) | Free T4 (ng/dL) |
|---|
| Serum Iron Conc. (μg/dL) | r-value | 0.425 | | | | |
| p-value | 0.001* | | | | |
| Thyroid Stimulating Hormone (TSH) (μIU/mL) | r-value | -0.342 | -0.635 | | | |
| p-value | 0.008* | <0.001* | | | |
| Free T3 (pg/mL) | r-value | 0.029 | 0.284 | -0.139 | | |
| p-value | 0.826 | 0.028* | 0.288 | | |
| Free T4 (ng/dL) | r-value | 0.023 | 0.060 | -0.125 | 0.104 | |
| p-value | 0.859 | 0.647 | 0.341 | 0.428 | |
T3: Triiodothyronine, T4: Thyroxine
* Statistically significant difference (p<0.05)
Correlations between RDW, MCV, Hb, haematocrit, RBCs versus TSH, FT3 and FT4 among the included patients.
| Variables | Thyroid stimulating hormone (μIU/mL) | Free T3 (pg/mL) | Free T4 (ng/dL) |
|---|
| Red cell Distribution Width (RDW) (%) | r-value | 0.500 | -0.221 | -0.071 |
| p-value | 0.000* | 0.089 | 0.590 |
| Mean Corpuscular Volume (MCV) (fl) | r-value | -0.333 | 0.241 | 0.066 |
| p-value | 0.009* | 0.063 | 0.619 |
| Haemoglobin (Hb) (gm/dL) | r-value | -0.714 | 0.241 | 0.164 |
| p-value | 0.000* | 0.063 | 0.210 |
| Haematocrit (%) | r-value | -0.586 | 0.237 | 0.195 |
| p-value | 0.000* | 0.068 | 0.135 |
| Red Blood Cell count (RBCs) (M/cumm) | r-value | -0.298 | 0.359 | 0.148 |
| p-value | 0.021* | 0.005* | 0.261 |
T3: Triiodothyronine, T4: Thyroxine
* Statistically significant difference (p<0.05)
Cut-off values of some blood indices and serum iron study parameters in predicting the occurrence of thyroid hypofunction (high TSH with normal or low FT4) in patients with IDA are presented in [Table/Fig-6], which showed that thyroid functions started to be affected in IDA patients when haemoglobin levels ≤8.3 gm/dL, serum ferritin ≤15.2 ng/mL, serum iron ≤19.4 μg/dL and TFS% ≤3.63.
Cut-off values of some blood indices and serum iron study parameters in predicting the occurrence of thyroid hypofunction (high TSH with normal or low FT4) in patients with iron deficiency anaemia.
| Variable | Cut-off | Sensitivity | Specificity | +PV* | -PV* | AUC* |
|---|
| Haemoglobin (gm/dL) | ≤8.3 | 58.82 | 88.37 | 66.7 | 84.4 | 0.806 |
| Mean Corpuscular Volume (MCV) (fl) | ≤58.5 | 52.94 | 90.70 | 69.2 | 83.0 | 0.720 |
| Red cell Distribution Width (RDW) (%) | >17.5 | 58.82 | 90.70 | 71.4 | 84.8 | 0.722 |
| Ferritin (ng/mL) | ≤15.2 | 94.12 | 39.53 | 38.1 | 94.4 | 0.659 |
| Serum iron (μg/dL) | ≤19.4 | 76.47 | 76.74 | 56.5 | 89.2 | 0.778 |
| Transferrin saturation% | ≤3.63 | 64.71 | 88.37 | 68.7 | 86.4 | 0.772 |
| Unsaturated iron binding capacity (μg/dL) | >483 | 64.71 | 74.42 | 50.0 | 84.2 | 0.665 |
| Total iron binding capacity (μg/dL) | >486 | 76.47 | 58.14 | 41.9 | 86.2 | 0.654 |
* +PV= positive predictive value, -PV= negative predictive value, AUC= area under the curve
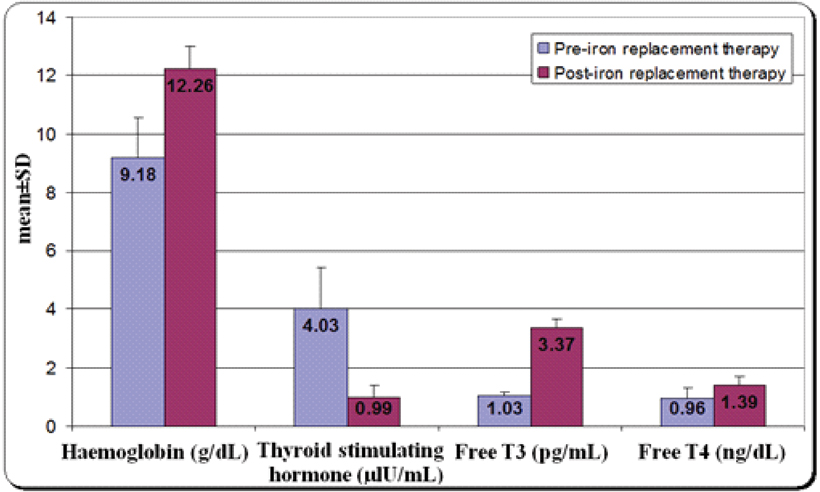
Comparison of the mean haemoglobin levels and thyroid profile (TSH, FT3 and FT4) among IDA patients pre and post iron replacement therapy are presented in [Table/Fig-7] which revealed significant decrease in the serum levels of TSH, with significant increase in the serum levels of FT3 and FT4 (p<0.001 for all).
Comparison of the mean haemoglobin levels and thyroid profile (TSH, FT3 and FT4) among iron deficiency anaemia patients pre- and post-iron replacement therapy.
TSH: Thyroid Stimulating Hormone, T3: Triiodothyronine, T4: Thyroxine
Discussion
The findings of the present study revealed significantly higher serum levels of TSH and significantly lower FT4 and FT3 among IDA children and with increasing the severity of IDA in comparison to the control group. In agreement with our findings, Kammal M and Abdrabo AA reported significant increase in the serum TSH levels among patients with IDA with non significant differences between patients and controls with regard to free T3 and free T4 levels, which means the presence of subclinical hypothyroidism in such patients [4]. Gökdeniz E et al., reported the occurrence of subclinical and primary hypothyroidism among IDA patients [21]. Also, a study done by Akhter S et al., reported significantly higher TSH levels and lower FT3 and FT4 among patients with low ferritin levels versus the control group [6]. A study done by Metwalley KA et al., revealed significantly higher TSH serum levels in children with IDA when compared with the control with significantly lower levels of serum FT3 and FT4 and significantly higher levels of serum TSH among children with severe IDA compared to those with mild to moderate IDA [22]. In disagreement with the present findings, a study done by Ipek I et al., reported no significant difference between the IDA group and control group with regard to thyroid profile [7]. These variations may be due to different geographic distribution, different demographic characteristics, inclusion criteria and the severity of anaemia of patients included in the study.
Thyroxine 5-deiodinase activity, which is responsible for peripheral T4 to T3 conversion is impaired in ID [23]. In the present study, there were significant increase in the TSH and significant lowering in the FT3 levels as the severity of anaemia increases with presence of significant positive correlation between serum iron levels and FT3. This could explain that apathy, lack of concentration, cognitive impairment, fatigue, lethargy, decreased physical activity and cold intolerance among iron deficient paediatric patients especially severe degree, could be attributed to the associated lower levels of thyroid hormones (thyroid hypofunction). This indicates that peripheral enzymatic conversion of T4 to T3 by thyroxine 5-deiodinase is iron dependent. So, FT3 decreases with decreasing iron serum levels and with increasing severity of anaemia; although, there was non significant decrease in the levels of FT4 with the severity of the anaemia. This indicates that IDA induced thyroid hypo function is of mild severity.
The findings of the present study revealed the presence of a significant negative correlation between TSH versus both ferritin and iron, with non significant correlation between ferritin versus either FT3 or FT4. In agreement with these findings, Kammal M and Abdrabo AA, reported a significant inverse correlation between serum TSH versus both iron and ferritin [4]. Bremner AP et al., reported inverse correlation between TSH and iron [24]. Other studies also reported significant negative correlation between TSH and ferritin. [16,22,25].
The findings of the present study also revealed significant positive correlation between FT3 and RBCs count with significant negative correlations of TSH and haemoglobin, haematocrit % and RBCs, supporting the association between thyroid hormones and erythropoiesis. In agreement with these findings, a study done by Khatiwada S et al., reported the presence of hypothyroidism among iron deficient children with negative correlation between TSH and haemoglobin [26]. Another study by Bremner AP et al., on relationships between thyroid hormones and erythrocyte parameters, reported significant positive correlation between FT3 and RBC count and they reported significant inverse relationships between TSH and each of haemoglobin, haematocrit and red cell counts [24]. On the contrary, Akhter S et al., reported that in iron deficient patients, haemoglobin levels and serum ferritin had no significant negative correlation with serum TSH [6].
To the best of our knowledge, no previous studies can be traced in the literature regarding the cut-off values of some blood indices and serum iron study parameters in predicting the occurrence of thyroid hypofunction (high TSH with normal or low FT4). The present study revealed that thyroid hypofunction can be predicted among children with IDA when haemoglobin levels ≤8.3 gm/dL, MCV ≤58.5 fl, RDW >17.5%, ferritin ≤15.2 ng/mL, iron ≤19.4 μg/dL, TFS ≤3.63%, unsaturated iron binding capacity >483 μg/dL and TIBC >486 μg/dL with the highest sensitivity was for ferritin and the highest specificities were for MCV, RDW and TFS% respectively.
The dosage of elemental iron required to treat IDA in children is 3 mg/kg/day, up to 60 mg/day for three months [27]. In the present study, TSH, FT3 and FT4 return to the euthyroid status according to the reference range, following three months of the oral iron replacement therapy (6 mg/kg/day divided into two doses). In accordance, Eftekhari MH et al., determined whether iron supplementation in iron deficient patients would improve thyroid function and found that iron supplementation improves thyroid function reporting a significant increase in FT4 in iron deficient children and adolescents [16]. Gökdeniz E et al., reported subclinical and primary hypothyroidism among patients with IDA with significant decrease in the TSH and rising in the FT4 following iron supplementation [21]. The literature also states that all patients with overt hypothyroidism and subclinical hypothyroidism with TSH >10 mIU/L should be treated with hormonal replacement therapy [20], while the mean level of serum TSH among IDA in the present study was 4.03±1.41 μIU/mL which did not indicate the need for thyroid hormone replacement therapy. On the contrary, Tienboon P and Unachak K, found no difference in FT4, FT3 and TSH levels in children with IDA before and after iron treatment [28], these difference can be attributed to difference in the number of cases enrolled, difference in the age and inclusion criteria.
Limitation
The relatively small sample size of the present study, indicates the need of future large scale studies to confirm the present finding. Also, the other limitation was the non involvement of children with other types of anaemia to evaluate the thyroid status, which require future studies.
Conclusion
The findings of the present study revealed the presence of thyroid hypofunction in the form of subclinical or primary hypothyroidism according to the severity of anaemia among children suffering from IDA, which is reversible once iron replacement therapy is started as early as possible to such patients, without the need to add thyroid hormone replacement therapy.
* Statistically significant difference (p<0.05)
T3: Triiodothyronine, T4: Thyroxine
* Statistically significant difference (p<0.05)
T3: Triiodothyronine, T4: Thyroxine
* Statistically significant difference (p<0.05)
** p1=mild anaemia versus moderate anaemia, p2=moderate anaemia versus severe anaemia, p3=mild anaemia versus severe anaemia
T3: Triiodothyronine, T4: Thyroxine
* Statistically significant difference (p<0.05)
T3: Triiodothyronine, T4: Thyroxine
* Statistically significant difference (p<0.05)
* +PV= positive predictive value, -PV= negative predictive value, AUC= area under the curve