Intestinal Sinonasal Adenocarcinoma with Distinct Immunophenotype
Monika Sharma1, Sabina Khan2, Shaan Khetrapal3, Sujata Jetley4, Abhinav Jain5
1 Demonstrator, Department of Pathology, Hamdard Institute of Medical Sciences and Research, Jamia Hamdard, Delhi, India.
2 Associate Professor, Department of Pathology, Hamdard Institute of Medical Sciences and Research, Jamia Hamdard, Delhi, India.
3 Assistant Professor, Department of Pathology, Hamdard Institute of Medical Sciences and Research, Jamia Hamdard, Delhi, India.
4 Professor and Head, Department of Pathology, Hamdard Institute of Medical Sciences and Research, Jamia Hamdard, Delhi, India.
5 Associate Professor, Department of Radiodiagnosis, Hamdard Institute of Medical Sciences and Research, Jamia Hamdard, Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sabina Khan, Associate Professor, Department of Pathology, Hamdard Institute of Medical Sciences and Research, Jamia Hamdard, Delhi-110062, India.
E-mail: drsabina1@gmail.com
Sinonasal adenocarcinomas are uncommon tumours accounting for 3% of all malignant tumours of the head and neck. They often present with symptoms of chronic nasal obstruction and recurrent epistaxis in patients with middle age to older age group. We report a case of intestinal sinonasal adenocarcinoma in a 60-year-old male which posed a diagnostic challenge due to its rarity, uncommon radiological presentation and distinct immunophenotype. Our results demonstrate that there exists a subset of intestinal sinonasal adenocarcinoma that is CK7-/CK20+.
Cytokeratin 7, Cytokeratin 20, Nasal Cavity
Case Report
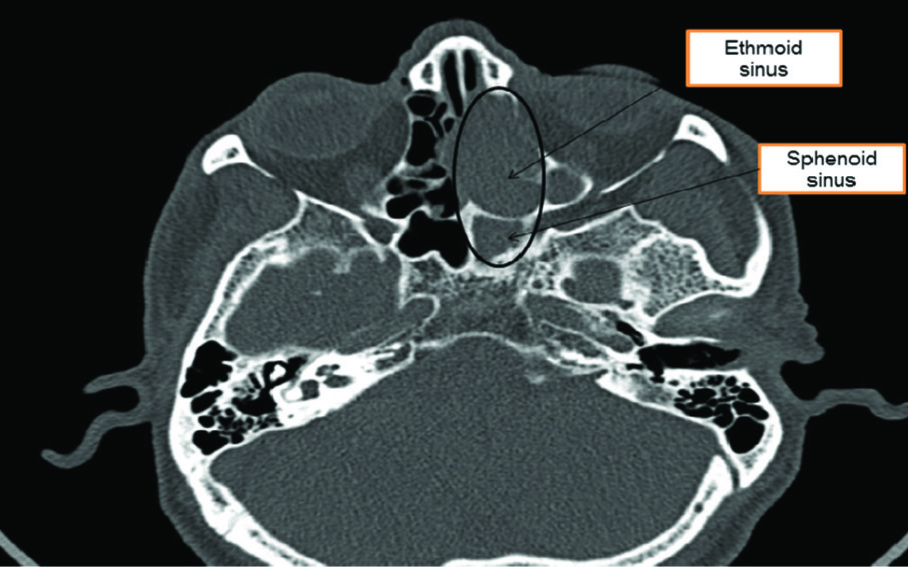
A 60-year-old male patient presented with heaviness, foul smelling discharge and obstruction in left nasal cavity for three to four months with one recent episode of bleeding from the nose. Examination showed a polypoidal mass with debris in left nostril. Complete blood count, liver and kidney function tests were within normal limits. No abdominal mass was found on further radiological investigations. X-ray paranasal sinus showed radiolucent area in left nasal cavity. On Computed Tomography (CT), a soft tissue mass was seen filling the ethmoid sinus and sphenoid sinus with bulging sinus walls and destruction of interlamellar septae along with thinning and destruction of lamina papyracea [Table/Fig-1]. Based on this, provisional clinical diagnosis of sinonasal polyposis was suggested.
CT findings of intestinal sinonasal adenocarcinoma located in ethmoid and sphenoid sinus with destruction of interlamellar septae.
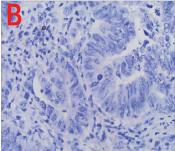
Patient underwent diagnostic nasal endoscopy and biopsy was done under local anaesthesia which was subsequently subjected for histopathological examination. Grossly, multiple grey white to grey brown soft tissue pieces altogether measuring 2 cm × 1.8 cm × 0.5 cm were received. Microscopy showed a tumour comprising of cells arranged in tubules and papillae lined by columnar cells. At places, stratification and nuclear crowding was also noted. Tumour cells showed moderate pleomorphism, enlarged vesicular nuclei, coarse chromatin with prominent nucleoli and scant to moderate amount of eosinophilic cytoplasm. Abnormal mitosis and necrosis was also seen [Table/Fig-2]. Based on the microscopic findings, a diagnosis of sinonasal adenocarcinoma, intestinal type was made. On immunohistochemistry, tumour cells were uniformly and strongly positive for Cytokeratin (CK) 20 [Table/Fig-3a] and negative for CK7 [Table/Fig-3b]. The patient underwent surgery followed by radiotherapy and was kept under close follow up for six months. Later patient was lost for follow up.
Tumour cells are composed of pseudostratified columnar cells with abundant eosinophilic cytoplasm (H&E 40X).
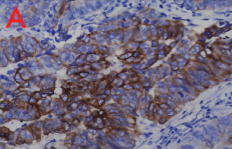
a) Immunostaining with CK20 shows a strong positivity (40X); b) Immunostaining with CK7 shows uniform negativity (40X).
Discussion
Sinonasal adenocarcinomas are unusual and account for approximately 0.1% of all malignancies. They are further divided into salivary and non-salivary gland type, with subdivision of non-salivary gland type as Intestinal Type Adenocarcinoma (ITAC) and non-intestinal type [1,2]. Intestinal type adenocarcinoma is second most common type of adenocarcinoma after adenoid cystic variant of salivary gland type adenocarcinomas [3]. Most commonly these tumours present with nasal symptoms such as obstruction, epistaxis and rhinorrhea with the involvement of the V2 branch of the trigeminal nerve reported in some cases [2]. Our patient also presented with nasal obstruction and single episode of epistaxis.
It is an aggressive malignancy seen mostly in males in an elderly age group and is associated with occupational exposure to nickel, wood and leather dust [1]. In our case, there was no such history of occupational exposure or association with viruses. Most common sites involved are ethmoidal sinus with left sided predominance followed by sphenoid sinus and frontal sinus [4]. Sinonasal carcinomas are usually diagnosed at late stages and present with extensive bone destruction. Surgery is often the mainstay treatment of these tumours with reported five year survival rates from 59% to 80% [5].
Different histological variants of Sinonasal carcinomas described are keratinizing and non keratinizing squamous cell carcinoma, cylindroid (transitional) cell carcinoma, verrucous carcinoma, basaloid squamous cell carcinoma, sarcomatoid (spindle cell) carcinoma, adenocarcinoma, small cell neuroendocrine carcinoma and undifferentiated (anaplastic) carcinoma. Squamous cell carcinoma is the most frequent type [4]. On the other hand, adenocarcinomas constitute only 10-20% of all malignancies of nasal cavity and paranasal sinuses [4]. Intestinal variant of sinonasal adenocarcinoma accounts for only 1-4% of total malignancies of head and neck region [6]. The site wise occurrence of ITAC is in the following order: ethmoid sinus (40%), nasal cavity (25%), maxillary antrum (23%) and indeterminate (9%) [6]. Adenocarcinomas are locally aggressive tumours with propensity to recur however, lymph node metastasis is rare [4].
Histological variants of ITAC are papillary, colonic, solid, mucinous and mixed. Papillary type has indolent course with best prognosis [6]. Solid and mucinous tumours are the most aggressive histological subtypes with short survival rate owing to their late presentation [7]. Local recurrence in ITAC is seen in more than 50% cases. Metastasis rarely occurs to lung, liver and bone [6]. On further work up, no evidence of metastasis was noted in our patient.
Differential diagnoses of ITACs are low-grade sinonasal adenocarcinomas and metastatic intestinal adenocarcinomas. ITAC cannot be distinguished with certainty from colorectal carcinoma metastatic to sinonasal tract on the basis of histology and immunophenotype alone. Although, colonic tumours have propensity to migrate to the sinonasal areas, even then they are far less common than primary sinonasal adenocarcinomas. In our case, metastatic adenocarcinoma was ruled out as no significant mass was found in the abdomen on further radiological investigations and colonoscopy was also normal [7]. ITACs must be properly distinguished from low-grade sinonasal adenocarcinomas because of the much less aggressive clinical course and a better prognosis of the latter. The distinction between low-grade sinonasal adenocarcinomas and ITACs is based on the higher grade of most ITACs and their predominant population of cylindrical cells and goblet cells along with frequent areas of necrosis, inflammation, occasional haemorrhage and 0 to 6 mitoses per high-power field [8]. Even then, sometimes it is difficult to distinguish these two.
Immunohistochemistry (IHC) is an important tool to distinguish ITAC from low grade sinonasal adenocarcinoma. The CK7 and CK20 profiles were reported to be most useful IHC markers in distinguishing ITACs from low grade sinonasal adenocarcinomas and nasopharyngeal adenocarcinomas [9,10]. Both non ITAC sinonasal and nasopharyngeal adenocarcinomas retained the CK7+/CK20- phenotype of surface respiratory epithelium and seromucous glands. Studies by Choi HR et al., and Bashir AA et al., found that most ITACs were positive for CK20, with a small proportion co-expressing CK7 and CK20, whereas all non-ITACs were consistently negative for CK20 and positive for CK7 [11,12].
Choi HR et al., found that some ITACs lack expression of CK7 as in their study they found that all their seven cases of ITAC were positive for CK20 [11]; however, four were negative for CK7 staining. Kennedy MT et al., and the published literature showed that CK20 was found to be consistently positive in all ITACs, and coexpression of CK7 and CK20 was found in most cases; however still a proportion of ITACs expresses a CK7-/CK20+ phenotype that is significant in number [9]. It is suggested that more rapid metaplastic transformation from respiratory epithelium to intestinal type epithelium leads to expression of both CK7 and CK20, however, a longer transition time accounts for CK7 staining to disappear and leaving positivity for only CK20 [11]. Our study showed results consistent with above mentioned studies. Thus, based on cytokeratin profiles, our results suggest that there exists a small population of ITAC that has a distinct phenotype of CK7-/CK20+.
Conclusion
The case is presented owing to its rarity and distinct immunophenotype. A high clinical suspicion of malignancy should always be made with a clinical history of epistaxis and clinico radiological mismatch in elderly. The merit of this case is that it highlights the distinctive phenotype of sinonasal ITACs, with all cases expressing CK20. Most ITACs also express CK7; however, there is a small but significant proportion of tumours that lack staining for CK7.
[1]. Leivo I, Update on sinonasal adenocarcinoma: classification and advances in immunophenotype and molecular genetic make-upHead Neck Pathol 2007 1(1):38-43. [Google Scholar]
[2]. Bhayani MK, Yilmaz T, Sweeney A, Calzada G, Roberts DB, Levine NB, Sinonasal adenocarcinoma: a 16-year experience at a single institutionHead Neck 2014 36(10):1490-96. [Google Scholar]
[3]. Leivo I, Sinonasal Adenocarcinoma: Update on classification, immunophenotype and molecular featuresHead Neck Pathol 2016 10(1):68-74. [Google Scholar]
[4]. Respiratory tract. In: Juan Rosai, EdRosai and Akerman’s Surgical Pathology 2009 Vol 19th editionMissouriMosby:309-11. [Google Scholar]
[5]. Hanna E, DeMonte F, Ibrahim S, Roberts D, Levine N, Kupferman M, Endoscopic resection of sinonasal cancers with and without craniotomy: oncologic resultsArch Otolaryngol Head Neck Surg 2009 135(12):1219-24. [Google Scholar]
[6]. Sklar EML, Pizarro JA, Sinonasal intestinal type adenocarcinoma - Involvement of the Paranasal SinusesAm J Neuroradiol 2003 24(6):1152-55. [Google Scholar]
[7]. Allende BV, Perez-Escuredo J, Martínez NF, Forcelledo MFF, Pendás JLL, Hermsen M, Intestinal-type Sinonasal Adenocarcinomas. Immunohistochemical Profile of 66 CasesActa otorrinolaringlogica 2013 64(2):115-23. [Google Scholar]
[8]. Barnes L, Intestinal-type adenocarcinoma of the nasal cavity and paranasal sinusesAm J Surg Pathol 1986 10(3):192-202. [Google Scholar]
[9]. Kennedy MT, Jordan RC, Berean KW, Perez-Ordonez B, Expression pattern of CK7, CK20, CDX-2, and villin in intestinal-type sinonasal adenocarcinomaJ Clin Pathol 2004 57(9):932-37. [Google Scholar]
[10]. McKinney CD, Mills SE, Franquemont DW, Sinonasal intestinal type adenocarcinoma: immunohistochemical profile and comparison with colonic adenocarcinomaMod Pathol 1995 8(4):421-26. [Google Scholar]
[11]. Choi HR, Sturgis EM, Rashid A, DeMonte F, Luna MA, Batsakis JG, Sinonasal adenocarcinoma: evidence for histogenetic divergence of the enteric and nonenteric phenotypesHum Pathol 2003 34(11):1101-07. [Google Scholar]
[12]. Bashir AA, Robinson RA, Benda JA, Smith RB, Sinonasal adenocarcinoma: immunohistochemical marking and expression of oncoproteinsHead Neck 2003 25(9):763-71. [Google Scholar]