Introduction
Cellular homeostasis is characterized by the balance between cell death and survival [1]. In our body normal cells follow a path of growth, division and death that is called apoptosis. The programmed cell death also known as apoptosis is synchronized through genetic control which is evolutionally controlled to disassemble cells. The process of apoptosis occurs in diverse physiological settings such as development, homeostasis of tissues, and maintenance of integrity of the organisms [2,3] and is also helpful for prevention of tumours or the spread of infectious diseases, as it enables elimination of damaged or infected cells. An abnormal progression of cells arising from malfunctions in the apoptotic pathways have been implicated for occurrence of various human diseases and has become one of the leading causes of cancers worldwide. Amongst the regulators of apoptosis are caspases, type of endoproteases, a few of which like caspases-1, 4, 5, 12 present in humans also control inflammation. These enzymes are produced as inactive zymogens which act through signalling events. They aggregate into dimers or macromolecular complexes after gaining catalytic activity. Caspases could be initiator caspases (caspase-8 and 9) or effector caspases (caspases-3, 6, and 7) depending on the mode of action [4].
Overexpression of anti-apoptotic proteins such as IAPs leads to defects in apoptosis (cell death) regulation and cancer development, as a result of malfunction and interference with apoptotic signalling via death receptors or intrinsic cell death pathways. IAPs, bind caspases as they have one to three common structures, the Baculovirus-IAP-Repeat (BIR)-domains [5]. Out of the eight human IAPs, XIAP is the most potent and best-defined anti-apoptotic IAP family member. XIAP selectively binds and inhibits caspases 3, 7 and 9, but not caspase-8. Thus, XIAP is an attractive target for novel therapeutic agents for the treatment of malignancies like the antisense oligonucleotides directed against XIAP, which are being evaluated in clinical trials and small molecule XIAP inhibitors, which are in preclinical development [6]. XIAP is found to be over-expressed in many cancer tissues. Increased XIAP levels have also been reported for ovarian carcinoma [7], B-cell Non-Hodgkin and Hodgkin lymphoma [8], clear cell renal cancer [9,10], oesophageal carcinoma [11], and non-small cell lung cancer [12]. The fact that XIAP is a significant biomarker and target for cancer therapy could not be generalized for all cancer tissues and its expression may not be always correlated with adverse clinical outcomes. This review is focussed on XIAP as a cancer biomarker with an objective to present an update for better understanding of current molecular and clinical aspects of XIAP expression in various cancers. The possibilities and current strategies of developing targeted drug therapy against XIAP or gene for both prognostic and diagnostic approach to manage cancer have also been presented.
Inhibitors of Apoptosis
Inhibitors of apoptosis were first identified in 1993 in the genome of Baculoviruses after genetic screening [13,14]. XIAP (BIRC4) is among the eight human IAP family members identified other than Neuronal Apoptosis Inhibitory Protein (NAIP) (BIRC1), c IAP1 (BIRC2), c IAP2 (BIRC3), survivin (BIRC5), Apollon/Bruce (BIRC6), Melanomas (ML) IAP (BIRC7 or livin) and Insulin-Like Peptide 2 (ILP 2) (BIRC8) [15]. IAPs are characterised by the presence of one to three BIR domains [16]. IAPs are responsible for negative regulation of programmed cell death or apoptosis. Research has revealed that IAPs can act either by directly inhibiting the caspases [17] or by preventing procaspase activation or other proteins needed to activate procaspases due to overexpression. Thus, the malfunctioning of the regulatory mechanism of cell proliferation and overexpression of IAPs causing cell resistance to apoptosis leads to transformation of normal cells into cancerous cells [18].
XIAP, a Novel Member of IAP Family
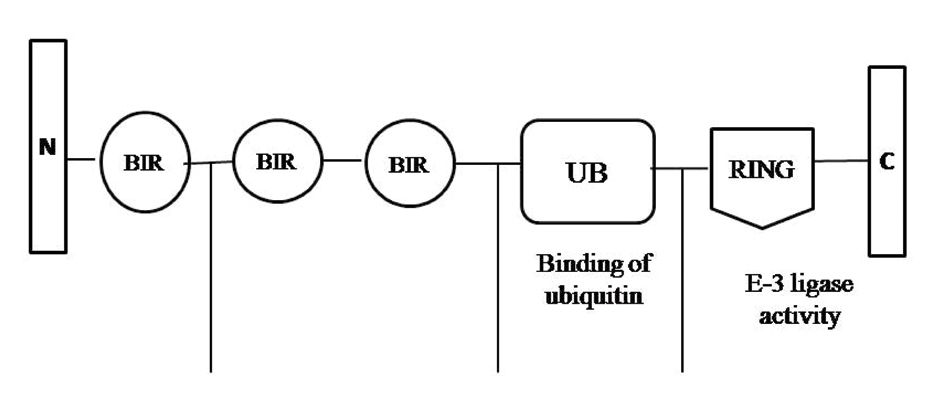
X-linked inhibitor of apoptosis protein also known as MIHA/hILP/BIRC4 is a novel member of the IAP family located on chromosome Xq25. XIAP is one of the most potent inhibitors of caspases -3, -7 and -9 and inhibits both extrinsic and intrinsic pathways of apoptosis. It is a 55 kDa, cytoplasmic protein, with three zinc-binding BIR domains (BIR 1–3) and a Really Interesting New Gene (RING) finger [19]. The BIR2 domain of XIAP inhibits caspases-3 and 7, while BIR3 binds to caspase-9 and inhibits the function of caspases [20]. The RING domain utilizes E3 ubiquitination ligase activity and enables IAPs to catalyse self-ubiquitination, caspase-3, or caspase-7 by reduction via proteasome activity [Table/Fig-1]. However, mutations affecting the RING Finger do not significantly affect apoptosis, because the BIR domain is sufficient for the protein’s function [21].
Structure of XIAP.
XIAP is a 57 k Da protein with three zinc-binding BIR domains (BIR 1–3) and RING-finger, it functions through binding to TRAF1 and TRAF2. The RING domain utilizes E3 ubiquitination ligase activity and enables IAPs to catalyze self-ubiquitination by reduction via proteasome activity.
N: amino terminal of XIAP, C: carboxyl terminal of XIAP, BIR: baculovirus inhibitor of apoptosis protein repeats, RING: Really interesting new gene, UB: Ubiquitin, TRF: Tumour necrosis factor receptor-associated factors.
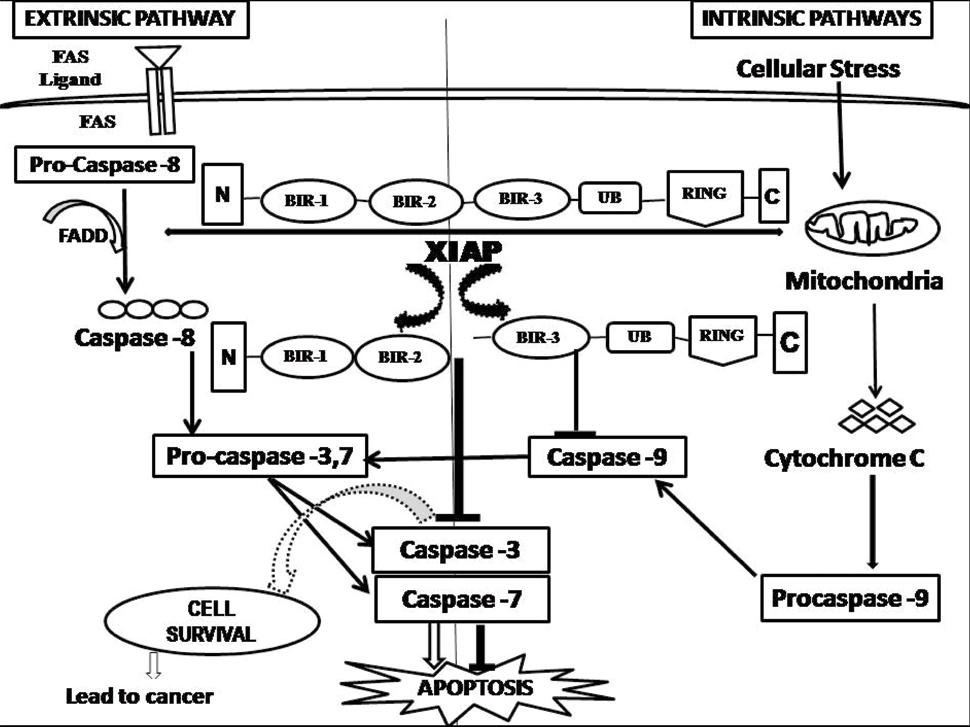
Intrinsic and Extrinsic Pathways-Signaling Mechanisms Associated with XIAP and Caspases
Two major pathways leading to apoptosis have been delineated: the extrinsic or receptor-mediated pathway and the intrinsic or mitochondrial pathway. Both extrinsic and intrinsic pathways in conjunction with caspases are responsible for the final cell death program [22]. As evident from [Table/Fig-2], the procaspase-8 with the help of Fas-Associated Protein with Death Domain (FADD) receptor gets activated as caspase-8 in the extrinsic pathway. Thus, both caspase 8 and caspase 9 through the apoptotic pathways activates caspase-3 and caspase-7 respectively. Activated caspase-3, 7 further executes the apoptosis process. XIAP binds with caspase-3, 7 by their BIR domain and inhibits the normal apoptosis process . Over expression of XIAP, member of IAP family leads to tumour genesis [23].
Role of XIAP in apoptotic pathways.
XIAP Overexpression in Cancer
X-linked inhibitor of apoptosis protein overexpression has been linked to cellular resistance to chemotherapy leading to non- response to drugs in many cancers. XIAP overexpression typically results in aggressive malignant behaviour of tumour, disease progression and poor prognosis [24,25,2]. This has led to research and development in cancer treatment by targeting XIAP with chemotherapy. The role of XIAP as a biomarker in cancer and various malignancies and target therapies is as follows:
a) Lung cancer: X-linked inhibitor of apoptosis protein is highly expressed in Non-Small Cell Lung Cancer (NSCLC) but not in normal lung tissue, which makes it an attractive target for lung cancer treatment [11,12]. It has been demonstrated through in vitro studies that combination of plasmid XIAP-shRNA and celecoxib (CXB) offer an effective therapeutic approach for NSCLC as compared to XIAP-shRNA and CXB alone. The mode of action is by suppression of proliferation, migration and invasion and induction of cell apoptosis. The fact that the combination suppressed the tumour growth was confirmed through studies in vivo mouse models. In another study, targeting the Bone Morphagenetic Protein (BMP) and Transforming Growth Factor-beta (TGFβ) type I and type II receptors caused a down regulation of XIAP, Transforming Growth Factor-beta Activated Kinase (TAK1), and Id1 leading to apoptosis of lung cancer cells [26]. Also, the prospective beneficial role of Antisense oligonucleotides targeting the XIAP gene product and the enhancement of the therapeutic efficacy of a cytotoxic agent vinorelbine (VNB) in a human NSCLC xenograft model has also been shown [27]. In the cytoplasm mSmac increases apoptosis, and XIAP inhibitsmSmac induced apoptosis by degradation of mSmac. Additionally, Second Mitochondrial Activator of Caspase (SMAC) Mimetics show their anticancer effectiveness in phase I and II clinical trials. The drug shows promise in the field of apoptosis-related drug development [28]. The studies suggest that suppression of XIAP, TAK1, and Id proteins may be required for a BMP inhibitor like DMH2 to induce significant death of cancer cells [29].
b) Pancreatic cancer: Pancreatic carcinoma is a leading cause of cancer deaths due to resistance to therapy. As compared to normal pancreatic ducts expression of XIAP is higher in pancreatic adenocarcinoma samples. Inhibitors of XIAP act in synergy with TRAIL by inducing apoptosis and inhibiting long-term clonogenic survival of pancreatic carcinoma cells [30]. One of the studies demonstrated that the simultaneous inhibition of XIAP and survivin expression considerably inhibited proliferation and increased apoptosis in pancreatic-1 cells. Also, stopping the expression of XIAP and survivin in pancreatic-1 cells reversed the Epithelial-Mesenchymal Transition (EMT) and subsequently increased chemosensitivity as well as decreased cell invasion and migration [31]. The therapeutic benefit of XIAP silencing with definite siRNA molecules or chemical compounds blocking XIAP is a ray of hope in this disease causing high mortality [32]. Use of SMAC mimetics, SW IV-134 and sensitising effect of XIAP interference toward gemcitabine on pancreatic cancer cell lines has also been studied [33].
c) Breast cancer: Breast cancer is ranked second leading cause of cancer related deaths all over the world in females after lung cancer. A study from Middle Eastern region explored prognostic and therapeutic role of XIAP in breast cancer and it was found that XIAP expression was significantly associated with tumour size, extranodal extensions and poorly differentiated breast cancer. Embelin and PI3-kinase inhibitor; LY294002 (IAP) were used to induce apoptosis and it was concluded that XIAP could be therapeutically targeted in treating breast cancers [34]. XIAP has been shown as an important biomarker of breast cancer and XIAP negative breast cancer patients have better prognosis. XIAP expression correlates significantly with hormone receptor expression [35]. It also acts as a potential therapeutic target for miR 200c which suppresses the tumour formation in breast cancer patients [36]. Overexpression of XIAP has been reported in varying extents in breast cancer cell lines or tumours and siRNA-based therapy targeted to XIAP is used for treatment [37]. A more recent study revealed that, though XIAP expression is higher in cancer tissue than normal tissue in breast cancer patients but the correlation between the survival, both disease-free and overall could not be established. More importantly in certain patient subgroups XIAP was a determinant of poor relapse-free survival and higher level of XIAP was a predictor of higher tumour recurrence [38].
d) Bladder cancer-XIAP and target therapies: Expression of XIAP increases in bladder cancer and causes resistance to chemotherapeutic drugs. When compared, well differentiated tumours expressed more XIAP than poorly differentiated ones. Considering the fact that XIAP inhibits caspase 9, the high expression of XIAP explains low caspase in these tumours [19,39]. Urinary XIAP has been studied as a marker for non-invasive diagnosis of Transitional Cell Carcinoma (TCC) of urinary bladder and increased expression of XIAP mRNA has been shown to be associated with stage and grade of cancer as well as higher expression was reported in tumour group as compared to controls [40]. Anti IAP therapy has been reported to be closely related to Epidermal Growth Factor Receptor (EGFR) expression. EGFR/XIAP overexpression has been used as therapeutic targets to improve clinical outcomes of bladder cancer patients. Anti-cancer activity of Embelin, an XIAP inhibitor has been studied and it has been recommended to develop Embelin as a chemotherapeutic agent to treat bladder cancer [41]. XIAP BIR domain has been also shown to strong positive regulator of EGFR expression in human bladder cancers by promoting EGFR protein translation [42].
e) Prostate cancer: X-linked inhibitor of apoptosis protein expression is an important therapeutic target and prognostic indicator in prostate cancer owing to its higher levels in cancerous cells as compared to normal tissue of prostate. The chances of tumour recurrence are much lower in patients with high levels of XIAP even when the tumour is high grade [43]. Being the most, common malignancy in males with androgen ablation established treatment approach; prostate cancer has gained attention for research to develop target therapy for aggressive drug resistant tumour cells which survive ablation. Combination therapy of new anti-androgen (CBDIV17) and embelin, and XIAP inhibitor have been found to be effective in terms of slowing of tumour growth and treating prostate cancers [44]. In order to identify patients of prostate cancer who could progress after radical prostectomy, XIAP, procaspase-3 and cleaved caspase-3 may help as biochemical markers and malignant behaviour of prostatic tumours could be predicted through XIAP and caspase expression [45]. Anoikis resistance in prostate cancer cells has been depicted to be result of an increased expression of XIAP [46]. Research on multidrug-resistance in prostate cancer showed that altered expression of IAP-2, XIAP, and survivin correlated with increased extent of cisplatin-resistance, making IAPs important in such clinical situations [47].
f) Renal cell carcinoma (RCC): Renal cell carcinoma is known to be resistant to chemotherapy and radiotherapy due to a high apoptotic threshold and XIAP expression has been designated as an independent prognostic marker in RCC. XIAP expression has been found to be high and significantly associated with tumour stages and the progression of clear cell RCCs . It has been seen to have an inverse relation with the tumour aggressiveness when survival was studied [6,10]. Relation of overexpression of XIAP and prognosis has been shown and it has been suggested that its down regulation may be helpful in dealing with immune resistance of tumours [48]. Molecular targeted therapy with Smac-N7 peptide has been suggested as a newer way to treat resistant RCC to therapeutic strategies triggering apoptosis [49].
g) Ovarian cancer: A study was conducted to evaluate the impact of XIAP expression on ovarian Clear Cell Carcinoma (CCC) that has a platinum-resistant phenotype. It was observed that XIAP expression further correlated with chemoresistance of primary chemotherapy [50]. XIAP associated factor 1 (XAF1) is known to be closely associated with anti-apoptosis and over growth of cancer cells. In ovarian cancer cells, there is role of XAF1 in suppressing XIAP expression and promoting cell death and thus, preventing invasion and leading to cisplatin sensitivity [51]. Research has shown the mechanism of dysregulation of XIAP in ovarian cancer by microRNAs (miRNAs). This has been seen as beneficial in terms of combating drug resistance in ovarian cancer and cisplatin-induced apoptosis and target the XIAP Three Prime Untranslated Reigon (3’UTR). MicroRNA-137 (miR-137) can sensitise ovarian cancer cells to have regulatory roles in various cellular processes, including apoptosis. In both, ovarian cancer cell lines and ovarian cancer tissues, there is an inverse relationship between miR-137 expression with the level of XIAP protein evident from the fact XIAP in SKOV3 cells can rescue the effect of miR-137 on apoptosis [52].
h) Oral cancer: The limited studies done, based on immunohistochemical analysis to assess the expression of XIAP in oral cancer, concluded that, the XIAP expression was spotted in cancer cells and was higher than that in normal cells and the relation could be with histological differentiation or pathological grades [53]. Earlier studies have explored the impact and expression of surviving a recently discovered IAP and found that in about 80% of oral Squamous Cell Cancers (SCC) the expression of surviving correlates with aggressive tumour phenotype while the normal oral mucosa did not express survivin [54]. A study on the expression of XIAP (anti-apoptotic marker) and its correlation with Ki-67 expression (proliferative marker) in benign and malignant salivary gland tumours concluded that one of the promising treatment options for metastatic, drug resistant salivary gland malignancies could be through reversal of XIAP actions. It has been suggested that continuing studies for other tumours also for evaluation of XIAP expression with larger sample size could throw light on breakthrough in combating the cancers through targeted therapy [55]. There is a paucity of studies in oral cancer patients in terms of XIAP expression and clinical stages, histological differentiation and classification of invasion mode of tumour cells and development of target therapies for the resistant and aggressive tumours needs to be studied.
i) Miscellaneous cancer: X-linked inhibitor of apoptosis protein expression and SMAC protein balance has been correlated with chemoresistant rectal cancers and it has been suggested that alternative neoadjuvant chemotherapy and radiotherapy could be the answers to management of resistant rectal cancers where XIAP is highly expressed [56]. XIAP downregulation in oesophageal cancers by RNAi in conjunction with chemotherapeutic drugs has been shown to sensitise cancer cell lines [57]. Clinical significance of XIAP and Nuclear Factor-βB (NF-βB) expression in oesophageal cancer patients and response to post-radical surgery, radiotherapy has been evaluated and positive correlations to progression/prognosis have been found [58]. In papillary thyroid cancers, XIAP overexpression has been therapeutically targeted with embelin and tumour extent size, age and poor outcomes are variables found to be significantly associated with XIAP expression signifying apoptotic value of XIAP as a cancer biomarker [59]. XIAP expression and its clinical significance has also been studied widely in liver cancers [60], cervical cancers [61] and B cell Non-Hodgkin and Hodgkin lymphoma [8]. The role of XIAP and targeted therapies in different cancers has been summarized in [Table/Fig-3].
Role of XIAP and targeted therapy in different cancers.
| S.No. | Type of Cancer | Role of XIAP | Targeted Therapy | Reference |
|---|
| 1. | Lung Cancer | XIAP has been shown to be highly expressed in lung cancer, but not in normal lung tissue, which makes it an attractive target for lung cancer treatment. | ShRNA combined with celecoxib, SMAC mimetics | [11,28] |
| 2. | Pancreatic Cancer | XIAP is highly expressed in pancreatic carcinoma and interfere with gemcitabine. | SMAC mimetic SW IV-134 | [33] |
| 3. | Breast Cancer | Comparatively highly expressed in breast cancer instead of normal tissue. (Considered a prognostic biomarker for basal-like breast cancer patients.) | Embelin and PI3-kinase inhibitor, miR 200c | [34,36] |
| 4. | Bladder Cancer | Overexpression of XIAP has been shown in tumour group and inhibit the chemotherapeutic drugs. | EGFR and XIAP acts as chemotherapeutic target for embelin. | [40] |
| 5. | Prostate Cancer | High level expression of XIAP in prostate cancer tissue is reported. | Embelin combined with anti-androgen (CBDIV17) is used for treatment by targeting XIAP. | [43] |
| 6. | Ovarian Cancer | XIAP expression is directly correlated with chemotherapeutic resistance and cisplatin induced apoptosis in ovarian cancer. | miR-137 via CRISPR/Cas9 inhibited apoptosis and upregulated XIAP. | [51] |
| 7. | Cervical Cancer | Overexpression of XIAP directly correlated with progression of cervical cancer. | miR-7 mediated growth suppression apoptosis induction. | [60] |
| 8. | Papillary Thyroid Carcinoma [PTC] | Significant correlation was found with overexpression of XIAP with tumour size in PTC. | Embelin and LY294002 induced a synergistic apoptotic response in PTC cells. | [58] |
Conclusion
X-linked inhibitor of apoptosis protein has become an important biomarker and therapeutic target as it is found to be overexpressed in many cancerous tissues but not in normal tissues. However, the expression and clinical outcomes, prognosis and predictive factors vary from cancer to cancer. There is a need for more molecular research and clinical studies to develop target chemotherapy for common cancers and patient subgroups that could be benefited so as to reduce the occurrence as well as mortality due to cancers worldwide. The fact that the expression of XIAP is not always associated with poor clinical outcomes poses challenge and creates the need for understanding the role and developing target drugs in personalized treatment of cancers. Thus, XIAP has the potential to serve as an attractive therapeutic target for treating a variety of malignancies by development of molecular and chemical XIAP inhibitors in conjunction with newer chemotherapies for resistant cancers.
[1]. Hanahan D, Weinberg RA, Hallmarks of cancer: the next generationCell 2011 144(5):646-74. [Google Scholar]
[2]. Raff M, Cell suicide for beginnersNature 1998 396(6707):119-22. [Google Scholar]
[3]. Ameisen JC, On the origin, evolution, and nature of programmed cell death. A timeline of four billion yearsCell Death Differ 2002 9(4):367-93. [Google Scholar]
[4]. Olsson M, Zhivotovsky B, Caspases and cancerCell Death Differ 2011 18(19):1441-49. [Google Scholar]
[5]. LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG, IAP-targeted therapies for cancerOncogene 2008 27(48):6252-75. [Google Scholar]
[6]. Obexer P, Ausserlechner MJ, X-linked inhibitor of apoptosis protein–a critical death resistance regulator and therapeutic target for personalized cancer therapyFront Oncol 2014 4:197 [Google Scholar]
[7]. Mansouri A, Zhang Q, Ridgway LD, Tian L, Claret FX, Cisplatin resistance in an ovarian carcinoma is associated with a defect in programmed cell death control through XIAP regulationOncol Res 2003 13(6-10):399-04. [Google Scholar]
[8]. Akyurek N, Ren Y, Rassidakis GZ, Schlette EJ, Medeiros LJ, Expression of inhibitor of apoptosis proteins in B-cell non-Hodgkin and Hodgkin lymphomasCancer 2006 107(8):1844-51. [Google Scholar]
[9]. Ramp U, Krieg T, Caliskan E, Mahotka C, Ebert T, Willers R, XIAP expression is an independent prognostic marker in clear-cell renal carcinomasHum Pathol 2004 35(8):1022-28. [Google Scholar]
[10]. Yan Y, Mahotka C, Heikaus S, Shibata T, Wethkamp N, Liebmann J, Disturbed balance of expression between XIAP and Smac/DIABLO during tumour progression in renal cell carcinomasBr J Cancer 2004 91(7):1349-57. [Google Scholar]
[11]. Zhang H, Li Z, Wang K, Ren P, Combined treatment of XIAP-targeting shRNA and celecoxib synergistically inhibits the tumor growth of non small cell lung cancer cells in vitro and in vivoOncol Rep 2015 33(3):1079-88. [Google Scholar]
[12]. Hofmann HS, Simm A, Hammer A, Expression of inhibitors of apoptosis (IAP) proteins in non-small cell human lung cancerJ Cancer Res Clin Oncol 2002 128(10):554-60. [Google Scholar]
[13]. Crook NE, Clem RJ, Miller LK, An apoptosis-inhibiting baculovirus gene with a zinc finger-like motifVirol 1993 67(4):2168-74. [Google Scholar]
[14]. Birnbaum MJ, Clem RJ, Miller LK, An apoptosis-inhibiting gene from a nuclear polyhedrosis virus encoding a polypeptide with Cys/His sequence motifsVirol 1994 68(4):2521-28. [Google Scholar]
[15]. Almagro de MC, Vucic D, The inhibitor of apoptosis (IAP) proteins are critical regulators of signaling pathways and targets for anti-cancer therapyExp Oncol 2012 34(3):200-11. [Google Scholar]
[16]. Clem RJ, Miller LK, Apoptosis reduces both the in vitro replication and the in vivo infectivity of a baculovirusJ Virol 1993 67(7):3730-38. [Google Scholar]
[17]. Roy N, Deveraux QL, Takahashi R, The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspasesEMBO J 1997 16(23):6914-25. [Google Scholar]
[18]. Seshagiri S, Miller LK, Baculo virus inhibitors of apoptosis (IAPs) block activation of Sf-caspase-1Proc-Natl Acad Sci-USA 1997 94(25):13606-11. [Google Scholar]
[19]. Deveraux QL, Leo E, Stennicke HR, Welsh K, Salvesen GS, Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspasesEMBO J 1999 18(19):5242-51. [Google Scholar]
[20]. Gewies A, Introduction to ApoptosisApo Review 2003 :01-26. [Google Scholar]
[21]. Duckett CS, Li F, Wang Y, Kevin J, Tomaselli Craig B, Armstrong R, Human IAP-like protein regulates programmed cell death downstream of Bcl-xL and cytochrome cMol-Cell-Biol 1998 18(1):608-15. [Google Scholar]
[22]. Budihardjo I, Oliver H, Lutter M, Luo X, Wang X, Biochemical pathways of caspase activation during apoptosisAnn Rev Cell Dev Biol 1999 15:269-290. [Google Scholar]
[23]. Harrington HA, Ho KL, Ghosh S, Tung KC, Construction and analysis of a modular model of caspase activation in apoptosisTheor Biol Med Modell 2008 5:26 [Google Scholar]
[24]. Muris JJ, Cillessen SA, Vos W, Houdt IS, Kummer J, Krieke JH, Immunohistochemical profiling of caspase signaling pathways predicts clinical response to chemotherapy in primary nodal diffuse large B-cell lymphomasBlood 2005 105(7):2916-23. [Google Scholar]
[25]. Parton M, Krajewski S, Smith I, Krajewska M, Archer C, Naito M, Coordinate expression of apoptosis-associated proteins in human breast cancer before and during chemotherapyClin Cancer Res 2002 8(7):2100-08. [Google Scholar]
[26]. Klingbeil O, Lesche R, Gelato KA, Haendler B, Lejeune P, Inhibition of BET bromodomain-dependent XIAP and FLIP expression sensitizes KRAS-mutated NSCLC to pro-apoptotic agentsCell Death Dis 2016 7(9):2365 [Google Scholar]
[27]. Hu Y, Cherton-Horvat G, Dragowska V, Baird S, Korneluk RG, Durkin JP, Antisense oligonucleotides targeting XIAP induce apoptosis and enhance chemotherapeutic activity against human lung cancer cells in vitro and in vivoLa Casse Clin Cancer Res 2003 9(7):2826-36. [Google Scholar]
[28]. Qin S, Yang C, Zhang B, Li X, Sun X, Li G, XIAP Inhibits Mature Smac-induced apoptosis by degrading it through ubiquitination in NSCLCInt J Oncol 2016 49(4):1289-96. [Google Scholar]
[29]. Augeri DJ, Langenfeld E, Castle M, Gilleran JA, Langenfeld J, Inhibition of BMP and of TGFβ receptors downregulates expression of XIAP and TAK1 leading to lung cancer cell deathMolecular Cancer 2016 :15-27. [Google Scholar]
[30]. Vogler M, Walczak H, Stadel D, Haas TL, Genze F, Jovanovic M, Small Molecule XIAP Inhibitors Enhance TRAIL-Induced Apoptosis and Antitumor Activity in Preclinical Models of Pancreatic CarcinomaCancer Res 2009 69(6):2425 [Google Scholar]
[31]. Yi X, Han T, Li Y, Long X, Li W, Simultaneous silencing of XIAP and survivin causes partial mesenchymal-epithelial transition of human pancreatic cancer cells via the PTEN/PI3K/Akt pathwayMol Med Rep 2015 12(1):601-08. [Google Scholar]
[32]. Li S, Sun J, Yang J, Zhang L, Wang L, Wang X, XIAP expression is associated with pancreatic carcinoma outcomeMol Clin Oncol 2013 1(2):305-08. [Google Scholar]
[33]. Hashim YM, Vangveravong S, Sankpal NV, Binder SP, Liu J, Goedegebuure SP, The targeted SMAC mimetic SW IV-134 is a strong enhancer of standard chemotherapy in pancreatic cancerJ Exp Clin Cancer Res 2017 36(1):14 [Google Scholar]
[34]. Hussain AR, Ahmed M, Bu R, Beg S, Alrashed AM, Melosantos R, XIAP overexpression is poor prognostic marker in breast cancer and can be targeted to induce efficient apoptosis (Abstract)In Proceedings of the 106th Annual Meeting of the American Association for Cancer Research 2015 Chicago, IL. Philadelphia (PA)AACR; Cancer Res:75 [Google Scholar]
[35]. Pandey M, Jha K, Kumar M, Shukla M, Shukla VK, XIAP Protein expression in Breast CancerWorld J Surg Med Radiat Oncol 2016 5:6-16. [Google Scholar]
[36]. Ren Y, Han X, Yu K, Sun S, Zhen L, Li Z, Mir-200c suppresses proliferation of breast cancer cellsMol Med Rep 2014 10(1):315-21. [Google Scholar]
[37]. Foster M, Thomas WO, Jolanta TH, Clarke RB, Brennan K, Bundred NJ, Targeting inhibitor of apoptosis proteins in combination with ErbB antagonists in breast cancerBreast Cancer Res 2009 11(3):R41 [Google Scholar]
[38]. Xu YC, Liu Q, Dai JQ, Yin ZQ, Tang L, Ma Y, Tissue microarray analysis of X-linked inhibitor of apoptosis (XIAP) expression in breast cancer patientsMed Oncol 2014 31(3):764 [Google Scholar]
[39]. Li M, Song T, Yin ZF, Na YQ, XIAP as a prognostic marker of early recurrence of nonmuscular invasive bladder cancerChin Med J 2007 120(6):469-73. [Google Scholar]
[40]. Srivastava AK, Singh PK, Singh D, Dalela D, Rath SK, Goel MM, Evaluation of urinary XIAP as a diagnostic biomarker of carcinoma of urinary bladderTumour Biol 2014 35(8):8243-48. [Google Scholar]
[41]. Fu X, Pang X, Qi H, Chen S, Li Y, Tan W, XIAP inhibitor Embelin inhibits bladder cancer survival and invasion in vitroClin Transl Oncol 2016 18(3):277-82. [Google Scholar]
[42]. Huang C, Zeng X, Jiang G, Liao X, Liu C, Li J, XIAP BIR domain suppresses miR-200a expression and subsequently promotes EGFR protein translation and anchorage-independent growth of bladder cancer cellJ of Hematol Oncol 2017 10(1):6 [Google Scholar]
[43]. Seligson DB, Hongo F, Huerta-Yepez S, Mizutani Y, Expression of X-linked inhibitor of apoptosis protein is a strong predictor of human prostate cancer recurrenceClin Cancer Res 2007 13(20):6056-63. [Google Scholar]
[44]. Danquah M, Duke CB, Patil R, Combination therapy of antiandrogen and XIAP inhibitor for treating advanced prostate cancerPharm Res 2012 29(8):2079-91. [Google Scholar]
[45]. Rodríguez-Berriguete G, Torrealba N, Ortega MA, Martinez-Onsurbe P, Olmedilla G, Paniagua R, Prognostic value of inhibitors of apoptosis proteins (IAPs) and caspases in prostate cancer: caspase-3 forms and XIAP predict biochemical progression after radical prostatectomyBMC Cancer 2015 15:809 [Google Scholar]
[46]. Berezovskaya O, Schimmer AD, Glinskii AB, Pinilla C, Hoffman RM, Reed JC, Increased expression of apoptosis inhibitor protein XIAP contributes to anoikis resistance of circulating human prostate cancer metastasis precursor cellsCancer Res 2005 65(6):2378-86. [Google Scholar]
[47]. Nomura T, Yamasaki M, Nomura Y, Mimata H, Expression of the inhibitors of apoptosis proteins in cisplatin-resistant prostate cancer cellsOncol Repor 2005 14(4):993-97. [Google Scholar]
[48]. Mizutani Y, Nakanishi H, Li YN, Matsubara H, Yamamoto K, Sato N, Overexpression of XIAP expression in renal cell carcinoma predicts a worse prognosisInt J Oncol 2007 30(4):919-26. [Google Scholar]
[49]. Bilim V, Yuuki K, Itoi T, Muto A, Kato T, Nagaoka A, Double inhibition of XIAP and Bcl-2 axis is beneficial for retrieving sensitivity of renal cell cancer to apoptosisBr J Cancer 2008 98(5):941-49. [Google Scholar]
[50]. Miyamoto M, Takano M, Iwaya K, Shinomiya N, Kato M, Aoyama T, X-chromosome-linked inhibitor of apoptosis as a key factor for chemoresistance in clear cell carcinoma of the ovaryBr J Cancer 2014 110(12):2881-86. [Google Scholar]
[51]. Zhao WJ, Deng BY, Wang XM, Miao Y, Wang JN, XIAP associated factor 1 (XAF1) represses expression of X-linked inhibitor of apoptosis protein (XIAP) and regulates invasion, cell cycle, apoptosis, and cisplatin sensitivity of ovarian carcinoma cellsAsian Pac J Cancer Prev 2015 16(6):2453-58. [Google Scholar]
[52]. Li X, Chen W, Zeng W, Wan C, Duan S, Jiang S, microRNA-137 promotes apoptosis in ovarian cancer cells via the regulation of XIAPBr J Cancer 2017 116(1):66-76. [Google Scholar]
[53]. Tamatani T, Takamaru N, Uchida D, Nagai H, Fujisawa K, Miyamoto Y, The expression of X-linked inhibitor of apoptosis in human oral squamous cell carcinoma and its relationship with clinical factorsIn: Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research 2012 72(8)Chicago, IL. Philadelphia (PA)AACR; Cancer Res:4954 [Google Scholar]
[54]. Lo M, Pannone G, Staibano S, Mignogna MD, Rubini C, Mariggiò MA, Survivin expression in oral squamous cell carcinomaBr J Cancer 2003 89(12):2244-48. [Google Scholar]
[55]. Heshiki W, Tomihara K, Yamazaki M, Arai N, Nakamori K, Noguchi M, Constitutive activation of caspase-3 in non-apoptotic oral squamous cell carcinoma cellsCancer Sci Ther 2015 7(2):75-80. [Google Scholar]
[56]. Flanagan L, Kehoe J, Fay J, Bacon O, Lindner AU, Kay EW, High levels of X-linked Inhibitor-of-Apoptosis Protein (XIAP) are indicative of radio chemotherapy resistance in rectal cancerRadiat Oncol (London, England) 2015 10:131 [Google Scholar]
[57]. Zhang S, Ding F, Lou A, Chen A, Yu Z, Ren S, XIAP is highly expressed in esophageal cancer and its downregulation by RNAi sensitizes esophageal carcinoma cell lines to chemotherapeuticsCancer Biol Ther 2007 6(6):973-80. [Google Scholar]
[58]. Zhou S, Ye W, Shao Q, Qi Y, Zhang M, Liang J, Prognostic significance of XIAP and NF-κB expression in esophageal carcinoma with postoperative radiotherapyWorld J Sur Oncol 2013 11:288 [Google Scholar]
[59]. Hussain AR, Bu R, Ahmed M, Jehan Z, Beg S, Sobhi AS, Role of X-linked inhibitor of apoptosis is a prognostic marker and therapeutic target in papillary thyroid carcinomaJ Clin Endocrinol Metab 2015 100(7):E974-85. [Google Scholar]
[60]. Shi YH, Ding WX, Zhou J, He JY, Xu Y, Gambotto AA, Expression of X-linked inhibitor-of-apoptosis protein in hepatocellular carcinoma promotes metastasis and tumor recurrenceHepatology 2008 48(2):497-507. [Google Scholar]
[61]. Liu S, Zhang P, Chen Z, Liu M, Li X, Tang H, MicroRNA-7 downregulates XIAP expression to suppress cell growth and promote apoptosis in cervical cancer cellsFEBS Lett 2013 587(14):2247-53. [Google Scholar]