Idiosyncratic Liver Injury due to Rivaroxaban
Aneesh Cherakulam Ratheendran1, Mukund A Prabhu2, Ku Natarajan3, Saritha Sekhar4
1 Research Fellow, Department of Adult Cardiology, Amrita Institute of Medical Sciences, Kochi, Kerala, India.
2 Assistant Professor, Department of Adult Cardiology, Amrita Institute of Medical Sciences, Kochi, Kerala, India.
3 Professor, Department of Adult Cardiology, Amrita Institute of Medical Sciences, Kochi, Kerala, India.
4 Associate Professor, Department of Adult Cardiology, Amrita Institute of Medical Sciences, Kochi, Kerala, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Aneesh Cherakulam Ratheendran, Cherakulam House, Opp. Tilak Club, Ayyappankavu, Chittoor Road, Kochi-682018, Kerala, India.
E-mail: aneeshcr13@gmail.com
Rivaroxaban is an oral direct factor Xa inhibitor that has been used for the primary and secondary prophylaxis of thromboembolic disorders. Once daily dosing makes it an attractive alternative when compared to other Novel Oral Anticoagulants (NOACS). Although, major and minor bleeding manifestations are commonly associated with rivaroxaban, hepatotoxicity has also been mentioned in the literature as a possible adverse effect. We report a case of drug induced liver injury in a 29-year-old male patient who was started on rivaroxaban for prophylaxis following an episode of Pulmonary Thromboembolism (PTE). There was a strong temporal relationship between the onset of symptoms and drug exposure in our case and the association was further validated by standard criteria. Roussel Uclaf Causality Assessment Method (RUCAM) score of 7 suggests rivaroxaban as the ‘probable’ culprit for drug induced liver injury in the present case. The temporal association of starting rivaroxaban and onset of symptoms along with elevated liver enzymes and bilirubin is a strong point in favour of drug induced liver injury due to rivaroxaban.
Drug induced liver, Factor Xa inhibitor, Newer oral anticoagulants, Pulmonary embolism
Case Report
A 29-year-old male with history of recent sclerotherapy treatment for varicose veins, was admitted with sub acute onset of breathlessness. Clinical and echocardiographic features were suggestive of PTE. Multidetector Computed Tomography (MDCT) pulmonary angiogram was done which confirmed the diagnosis [Table/Fig-1].
Multidetector computed tomography pulmonary angiogram.
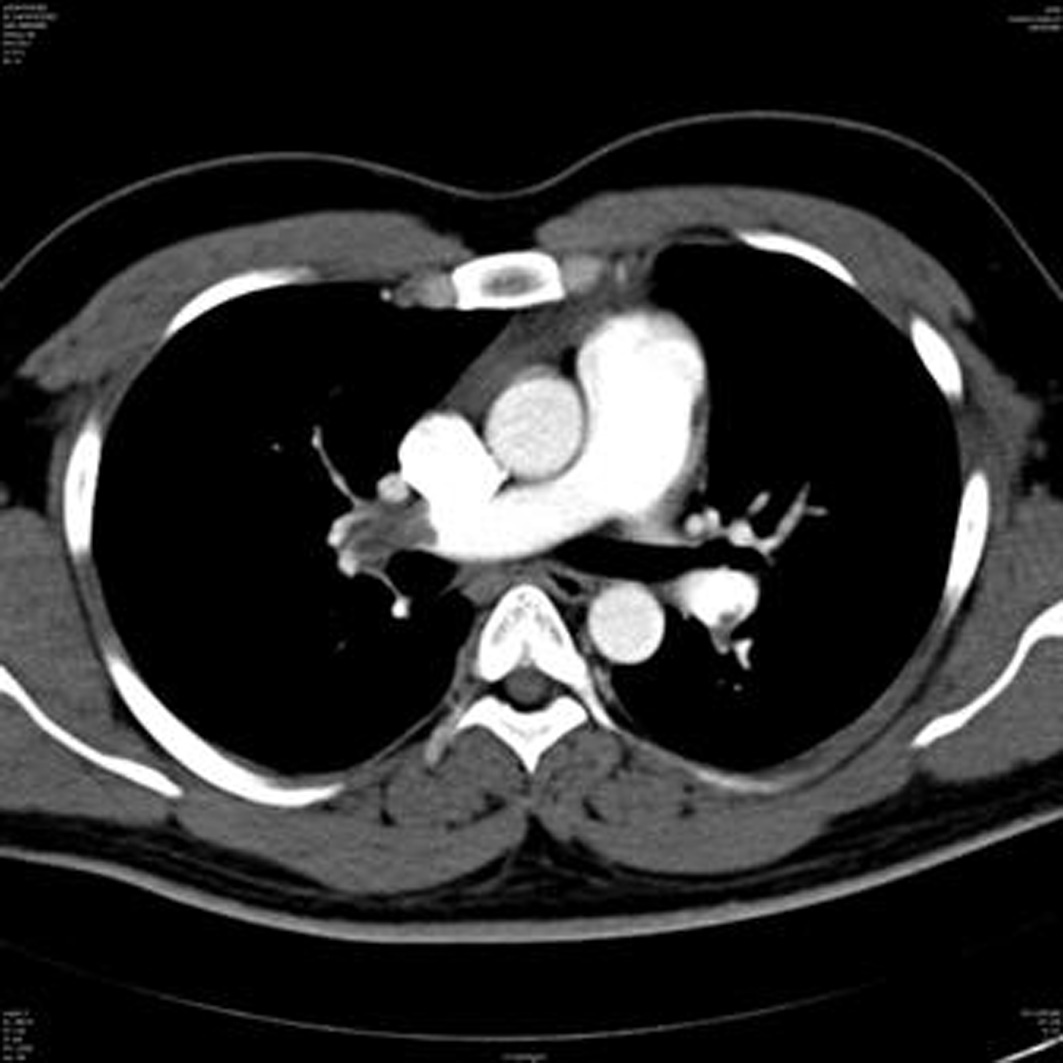
Thrombolytic therapy was initiated with streptokinase 250,000 units IV bolus over 30 minutes followed by infusion of 100,000 units per hour for 24 hours and following this, patient clinically improved. Prophylactic anticoagulation was started with rivaroxaban (15 mg twice daily) after thrombolytic therapy, overlapping with heparin for a day. Baseline laboratory values on admission showed total Bilirubin -1.0 mg/dL (Direct-0.18 mg/dL); Aspartate Aminotransferase (AST)-34.5 IU/L; Alanine Aminotransferase (ALT)-45 IU/L; Alkaline Phosphatase (ALP)-80 IU/L.
On the third day of starting rivaroxaban, patient started having abdominal pain, vomiting. His Liver Function Tests (LFT) showed total bilirubin -4.58 mg/dL [Table/Fig-2], AST-193 IU/L; ALT-188.9 IU/L, ALP-127 IU/L. However, there were no signs of encephalopathy. Ultrasound scan of the abdomen did not reveal any abnormality and there was no evidence of any biliary duct obstruction. Clinical and laboratory features were suggestive of possible drug induced liver injury due to rivaroxaban.
Rise and fall of total bilirubin (T.Bil).
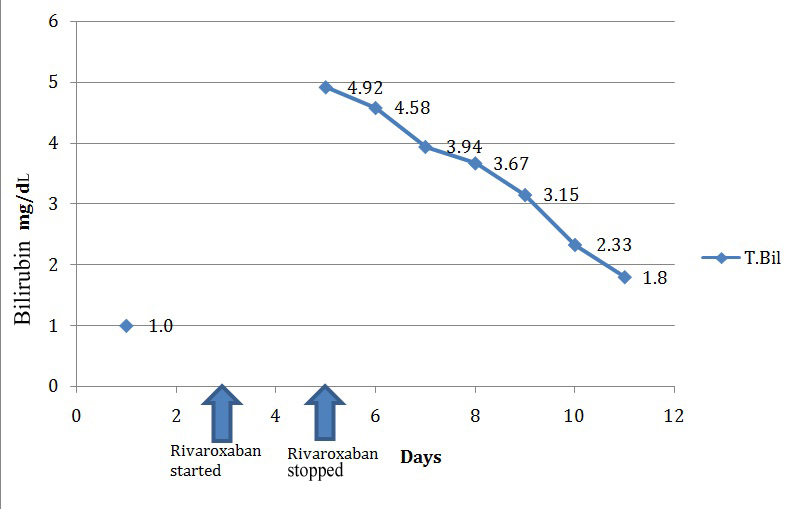
Patient did not have prior history of chronic liver disease, alcohol abuse or recent intake of any other hepatotoxic medicines. Viral hepatitis panel was negative. The only other medication co-administered was pantoprazole.
Rivaroxaban was withdrawn and heparin restarted. Liver enzymes continued to rise for two days even after stopping rivaroxaban after which it started to show a consistent decline. First to show a falling trend was SGOT, followed by SGPT, and finally ALP started to decline on the 5th day after stopping the drug [Table/Fig-3]. There was associated clinical improvement as well.
Rise and fall of liver enzymes.
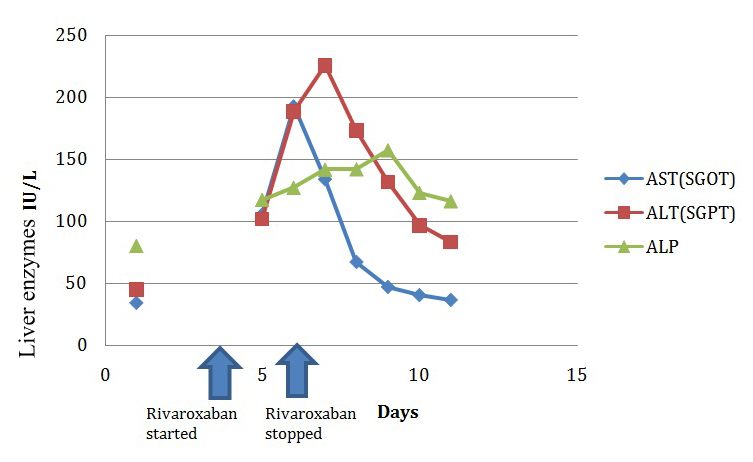
Patient was initiated on Warfarin once the liver enzymes improved, and the dose was titrated according to International Normalised Ratio (INR). LFT values subsequently normalised. At two months follow up, he was maintaining the target INR and the liver function tests were normal. The patient was later started on another newer anticoagulant Dabigatran which is a direct thrombin inhibitor and he was tolerating the drug well.
Discussion
Rivaroxaban is a selective, factor Xa inhibitor that is approved for the prevention of systemic embolism in patients with nonvalvular atrial fibrillation, prevention of deep vein thrombosis after orthopaedic surgery, and for the treatment of deep vein thrombosis and/or pulmonary embolism [1]. Once daily dosing makes it an attractive alternative when compared to other NOACS. The incidence of major and non-major bleeding has always received attention in the various landmark trials of rivaroxaban, while data regarding safety profile of the drug relating to other organ systems are scanty. The combination of ALT level of more than three times the Upper Limit of Normal (ULN) range and a bilirubin level of more than twice the ULN was observed in 0.2% of study population in the rivaroxaban group in the EINSTEIN PE Trial [2]. Apart from trial statistics real world data regarding hepatic side effects are still lacking. This case report describes a patient who developed Drug Induced Liver Injury (DILI) after initiation of rivaroxaban.
RUCAM score of 7 [Table/Fig-4] suggests rivaroxaban as the ‘probable’ culprit for drug induced liver injury in the present case [3]. The temporal association of starting rivaroxaban and onset of symptoms along with elevated liver enzymes and bilirubin is a strong point in favour of drug induced liver injury due to rivaroxaban. The consequent decline of transaminase values on withdrawing the drug is further supportive.
RUCAM causality assessment method patients score (interpretation).
| Pattern of liver injury | Hepatocellular |
|---|
| Temporal relationship of initiation of drug with liver injury |
| a) Drug start | 1 |
| b) Drug stop | 1 |
| Course after drug cessation | 3 |
| Risk factors, alcohol | 0 |
| Risk factors; age | 0 |
| Concomitant drugs | 0 |
| Exclusion of other causes of liver injury | 1 |
| Previous information on hepatotoxicity of the drug | 1 |
| Response to re-administration of a drug | 0 |
| Total score | 7 |
| Score interpretation | Probable |
Russmann S et al., has reported 14 cases of liver injury associated with Rivaroxaban which was gathered from multiple pharmacovigilance database [4]. About 13 out of 14 patients showed recovery following discontinuation of drug while one patient died of some other cause. Baig M et al., has reported a case of acute liver failure after initiation of rivaroxaban [5]. Although aminotransferase levels started to decline rapidly after with holding rivaroxaban in this case [5], the patient died of multiorgan failure. In our case the patient started developing features of drug induced liver Injury within three days of starting treatment. Only one patient in previous reports [4] had such early development of liver injury. There is only one prospective study which showed risk of liver injury among NOACS (rivaroxaban, apixaban and dabigatran) in patients with atrial fibrillation by Alonso A et al., and this showed a lower risk of liver injury with NOACS when compared to warfarin. Among NOACS rivaroxaban had the highest risk while dabigatran showed the lowest risk which probably gives the explanation why our patient tolerated dabigatran well [6].
Conclusion
Clinicians tend to focus more on major and minor bleeding associated with anticoagulant drugs which have hepatic and other systemic side effects as well. Although not a common side effect, clinicians should watch for drug induced liver injury in patients who are initiated on rivaroxaban. Early identification and prompt withdrawal of the drug can help in recovery without any residual effects. More detailed post marketing data on liver injury associated with rivaroxaban are required to understand the safety profile of the drug. This is particularly relevant as the use of rivaroxaban, with its attractive advantage of lack of need to monitor and expanding indications for use, is anticipated to increase in clinical practice. Other NOACS like dabigatran may be used safely in patients who develop rivaroxaban induced hepatotoxicity.
[1]. Harder S, Graff J, Novel oral anticoagulants. Clinical pharmacology indication and practical considerationsEur J Clin Pharmacol 2013 69(9):1617-33. [Google Scholar]
[2]. The EINSTEIN–PE InvestigatorsOral rivaroxaban for the treatment of symptomatic pulmonary embolismN Engl J Med 2012 366(1):1287-97. [Google Scholar]
[3]. Danan G, Benichou C, Causality assessment of adverse reactions to drugs - I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuriesJ Clin Epidemiol 1993 46(1):1323-30. [Google Scholar]
[4]. Russmann S, Niedrig D, Budmiger M, Schmidt C, Stieger B, Hürlimann S, Rivaroxaban postmarketing risk of liver injuryJ Hepatol 2014 61(1):293-300. [Google Scholar]
[5]. Baig M, Wool KJ, Halanych JH, Sarmad RA, Acute liver failure after initiation of rivaroxaban: a case report and review of the literatureNorth Am J Med Sci 2015 7(15):407-10. [Google Scholar]
[6]. Alonso A, MacLehose RF, Chen LY, Lindsay GS, Bengtson LG, Alanna M, Prospective study of oral anticoagulants and risk of liver injury in patients with atrial fibrillationHeart 2017 103(11):834-39. [Google Scholar]