ECC is defined as the incidence of one or more decayed, filled tooth surfaces or missing (due to dental caries) in any primary tooth in a child 71 months of age or younger [1]. Untreated dental caries is a major problem of public health in many countries around the world, and more specifically untreated caries in deciduous teeth is one of the most prevalent conditions affecting millions of children throughout the world [2]. Obviously if left untreated, dental decays may finally result in severe pain and infections [3] which can affect the child’s performance and school attendance [4]. This will obligate the clinician to perform invasive interventions rather than control the lesions with earlier non-invasive treatment [5].
In this regard, a number of chemical products including various dentifrices, rinses, varnishes, solutions and gels which contain chlorhexidine, active calcium and phosphate particles, metals and fluoride ions are used extensively as non-invasive treatment to decrease caries progression [6].
Fluoride products like Amine Fluoride (AmF), Stannous Fluoride (SnF2), Silver Diamine Fluoride (SDF), TiF4 and Sodium Fluoride (NaF) have been proved to possess a significant remineralising effect on dental caries [6-9]. NaF potently reduces the solubility of mineralised tooth components [10,11]. Semiannual topical application of NaF in high concentration (5%) provides an average reduction of 26% in caries of permanent teeth of children in non-fluoridated areas. It is now widely used as varnish in more than 40 countries around the world, particularly throughout Europe, Australia, the Middle East and Asia [12].
However, despite the high efficacy of NaF varnish in preventing initial lesions, it does not have an impressive effect on deeper lesions penetrating through enamel layer [13].
TiF4 has become popular recently in the dental research as a preventive agent against dental caries [14-17]. Some studies comparing the protective effect of TiF4 versus NaF have concluded that the experimental TiF4 is more effective in enamel remineralisation in vitro [18] and causes more reduction in enamel demineralisation in situ [19]. Higher uptake and deeper penetration of fluoride and lower acid solubility of tooth structure have been shown with application if TiF4 in comparison to NaF [20]. These results suggest its use for prevention of caries in man; however, its cytotoxicity is still questionable.
According to the current data, concentration, pH and time of application are the three main factors which would influence the cytotoxic effect of TiF4 on oral cells and subsequently its safety for clinical use [21].
Finding out the optimal operational conditions for Ti-based solutions can improve the efficacy and safety of this preventive agent and will economise the circumstances predisposing its use in dental clinics.
In this study, we tried to find out the best and safest operational environment for the application of TiF4 solutions. Moreover, RSM is introduced to dentistry world as a novel pattern for analysing interactions of multiple factors simultaneously without consuming excessive time and energy [22]. Results of this study would bring a proper guide for clinicians to apply titanium fluoride safely, and for dental researchers to learn effective utilisation of RSM to improve dental science and materials.
Materials and Methods
This experimental study was performed at cell culture laboratory at Pharmaceutical Biotechnology Department, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran, during August 2016 to December 2016 and approved by a Local Ethic Committee in Shiraz University of Medical Sciences, Shiraz, Iran (#95-01-03-12523).
Preparation of fibroblast cells: HGF1-PI 1 coding as C165 were obtained from Pasteur Institute of Iran (IPI). It was transferred to the laboratory in a pack of dry ice. The master cell bank consisted of 1 mL HGF1-PI 1. Vial including 1 mL HGF1-PI 1 was transferred to a 96-well plate including Dulbecco’s Modified Eagle Medium (DMEM) cell culture which consisted of 10% Fetal Bovine Serum (FBS), 100 U/mL penicillin and 100 mg/mL streptomycin in an aseptic condition with 5% CO2 and 100% humidity at 37°C. Each well had the maximal volume of 323 μL in which the volume of every well was set to 200 μL. Cells were subcultured three times before performing the experiment in order to allow the cells to adapt with the laboratory environment. Finally, in third step of cell culture, the concentration of fibroblast-like cells reached at about 1×104 cells per well. After this stage which lasted for 24 hours, cells were ready to be exposed to TiF4 solution in different concentration, pH and time displayed in [Table/Fig-1] and in a manner which CCD designed. In the next step, cells were exposed to tetrazolium MTT (3-{4, 5-dimethylthiazolyl-2}-2,5-diphenyltetrazolium bromide).
Independent variables; their coded and actual values used for optimisation study.
| Independent variable | Units | Symbol | | Code levels | |
|---|
| | | -1 | 0 | 1 |
| concentration | % | X1 | 1 | 2 | 4 |
| pH | | X2 | 1 | 1.5 | 2 |
| time | min | X3 | 0.5 | 2.5 | 5 |
Experimental design: In the preliminary studies, concentration, pH and time of application of TiF4 solution seemed to be three critical factors influencing cell viability [21]. In this regard, [Table/Fig-1] displays formerly studied range of values for each parameter in brief. Concentration of TiF4 solution extended from 1% to 4%; pH values limited between 1 and 2, and time of application ranged from 0.5 to 5 minutes [21,23-25]. The optimised response was the amount of cell viability (%).
Optimisation study using RSM: The optimal operational conditions after TiF4’s application on HGF1-PI 1 were estimated using the statistical package Design-Expert, version 7.0.0 (Stat- Ease, Inc. Minneapolis, MN, USA). To evaluate the inter-action and quadratic effects of three independent factors {concentration (X1), pH (X2), and time (X3)} on cell viability (Y), the Composite Central Design (CCD) was used. The final design contained 20 experimental points implemented in a random order, including 14 factorial and 6 central points. Six replicates (runs 3, 6, 10, 14, 17, 20) at the center of the design were performed to compute the pure error sum of squares. To predict the percentage of cell viability (Y), a regression model was estimated. The Response (Y) is equal to some linear, quadratic and interactive terms as below:
Y = β0 + ∑ βi xi + ∑ βii xii2 + ∑ βij xixj + ε (Eq. 1)In Eq. 1, β0, Bi, Bii and Bij stand for the constant, linear, quadratic and the cross-product coefficients respectively. Moreover, the studied independent variables indicated as Xi and Xj, and ε signified the residual error value.
The F-distribution analysis carried out to calculate the statistical significance of the estimated regression model. To assess the fitting of the model, R2 coefficient was calculated.
At the end, in order to study the effects of variations in parameter levels on cell viability and to determine the optimum level for each factor, the response surface curves were designed.
Evaluation of cell viability using MTT assay: This method measures conversion of the tetrazolium MTT salt into formazan as a marker for mitochondrial activity which constantly occurs in most viable cells. About 5 mg/mL tetrazolium MTT solution was added to each well and the cells were incubated for one hour. Then 100 μL dimethyl sulfoxide (DMSO) was charged into the wells to make the purple formazan soluble. The quantity of formazan was detected by a plate reader at 570 nm and measured as Optical Density (OD) [26]. Percentage of cell viability was reported using this formula:
Cell viability = OD 570 (test)/ OD 570 (control) × 100% (Eq. 2)All the experiments were repeated three times and the average numbers were recorded.
Results
Data Analysis and Evaluation of the Models
The CCD is able to present the information in forms of curvature descriptions. It can also model lack of fit. Moreover, the computed measurements using CCD are reproducible [27].
After preliminary studies, a RSM of CCD including three factors (concentration, pH and time) and their respective three levels displayed in [Table/Fig-1] was applied to evaluate the effects of the mentioned factors.
The DoE and the obtained results were shown in [Table/Fig-2]. The regression model to predict the percentage of cell viability (Y) was presented by the following equation (using actual values):
Statistical data analysis of the evaluation of studied parameters on cell viability including analysis of variance (ANOVA) and also the regression coefficients.
| Source | DF* | Cell viability(%) (Sum of squares) | Mean square | F-value | p-value |
|---|
| Model | 9 | 4871.36 | 541.26 | 7.65 | 0.0019 |
| X1 | 1 | 1429.57 | 1429.57 | 20.20 | 0.0012 |
| X2 | 1 | 436.70 | 436.70 | 6.17 | 0.0323 |
| X3 | 1 | 2062.15 | 2062.15 | 29.14 | 0.0003 |
| X1 X2 | 1 | 3.13 | 3.13 | 0.044 | 0.8378 |
| X1 X3 | 1 | 55.13 | 55.13 | 0.78 | 0.3982 |
| X2 X3 | 1 | 105.13 | 105.13 | 1.49 | 0.2509 |
| X12 | 1 | 107.05 | 107.05 | 1.51 | 0.2469 |
| X22 | 1 | 416.67 | 416.67 | 5.89 | 0.0357 |
| X32 | 1 | 389.72 | 389.72 | 5.51 | 0.0409 |
| Residual | 10 | 707.59 | 70.76 | | |
| Lack of fit | 5 | 702.75 | 140.55 | 145.40 | < 0.0001 |
| Pure error | 5 | 4.83 | 0.97 | | |
| Cor. total | 19 | 5578.95 | | | |
| SD | | 8.41 | | | |
| Mean | | 67.95 | | | |
| CV % | | 12.38 | | | |
| PRESS† | | 5465.84 | | | |
| R2 | | 0.8732 | | | |
| R2adj | | 0.7590 | | | |
| R2pred | | 0.0203 | | | |
| Adeq. precision | | 9.473 | | | |
* DF: Degree of freedom.
† PRESS: Predicted residual sums of squares
Y = - 4.15322 X1+82.61207 X2+3.07720 X3 + 0.83333 X1X2 + 0.77778 X1X3 – 3.22222 X2X3 – 1.21129 X12 – 21.50825 X22 – 1.02722 X32 (Eq. 3)In Eq. (3), Y is the percentage of cell viability and correspondingly, X1, X2 and X3 stood for the coded variables of the concentration, pH and time of application of TiF4 solution. Results of the analysis of variance (ANOVA) for experimental data were offered in [Table/Fig-2].
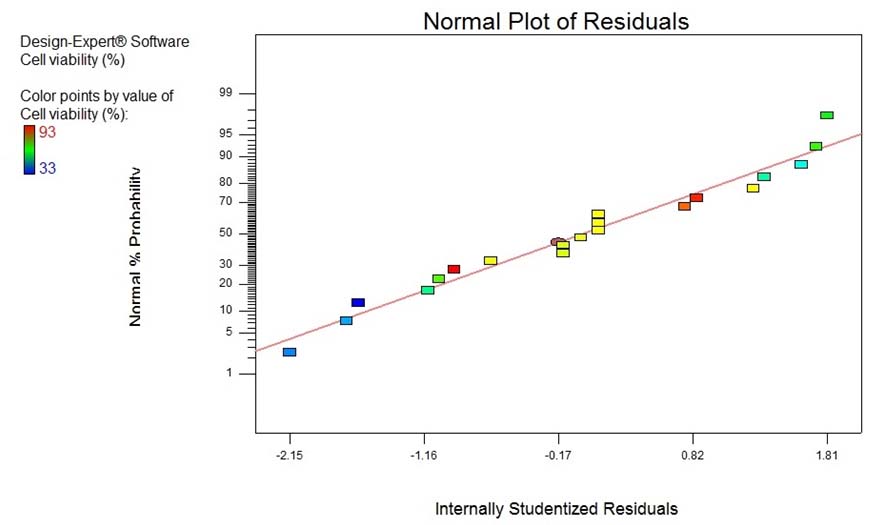
As displayed in [Table/Fig-2], the R2 value, adjusted R2 value (R2adj) and predicted R2 value (R2pred) found to be 0.8732, 0.7590 and 0.0203, respectively, which demonstrate that the regression model for cell viability fits to the experimental measurements [Table/Fig-3].
Predictability of the obtained model for cell viability (%) was studied using the scatter plot of the predicted versus measured experimental values.
Graphical Interpretation of the Response Surface Model
As represented in [Table/Fig-4a-c], to determine the optimum level for each factor, Three-Dimensional (3D) surface plots and contour plots were made. The 3D surface plots show the influence and dealings of two independent parameters on the cell viability while the other independent parameter is fixed.
Response surface plots (3D) showing the influence of three studied variables on fibroblast cell viability (%), when optimising the following pair of parameters, while the other parameter was kept constant at a central point (zero level). The interaction between concentration and pH levels on cell viability are presented as a; interaction between concentration and time on the studied response is depicted as b; whilst c presents the interactions between pH and time.
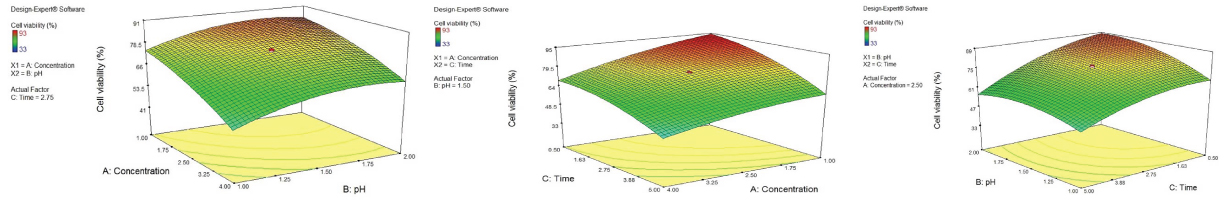
As shown in [Table/Fig-4a], elevating concentration level resulted in the reduction of cell viability with a slight slope initially, followed by a sharper inclination passing approximately the point indicating 1.75% concentration of the solution. The trend of decreasing cell viability with raising the concentration appeared to change in a milder manner, considering the pH value of 2.0 (in comparison to 1.0).
The [Table/Fig-4a] also showed that increasing the level of pH value favored the percentage of cell viability. A similar trend was seen for the same factor relationships (pH and cell viability) in [Table/Fig-4c].
[Table/Fig-4b] displaying the interaction of concentration and time and their effects on cell viability, demonstrated that with increasing time of application from 0.5 to 5 minutes the cell viability percentage decreased. A similar trend could be seen in [Table/Fig-4c], depicting the influence of time and pH factors on cell viability. Following the trend, at the point of 1% concentration, the cell viability would be at its best percentage (about 95%) when the time reaches at its least amount (0.5 minute) [Table/Fig-4b].
Discussion
In the present study, the study parameters were selected after searching and studying the existing literature on TiF4’s application in dentistry. After accurate exploration of the literature, we found that these three factors (concentration, pH and time) are the main parameters influencing the preventive effect of this fluoride product. Upon literature search, we found different studies which had evaluated the efficacy of TiF4 as a preventive agent at 1%, 1.55%, 3.4% and 4% concentration. Based on the preliminary studies, we selected 1% concentration, pH 1 and 0.5 minute as the lower limits and 4% concentration, pH 2 and 5 minutes as the upper limits of the ranges of the studied factors.
[Table/Fig-4a] demonstrated the effect of concentration, pH and their interaction on cell viability. The trend says that from 1 to approximately 2% TiF4 solution, the concentration factor had a minimal cytotoxic effect comparing thicker TiF4 solutions which seemed to have higher influence on cell viability.
In an animal study, Reed AJ and Bibby BJ demonstrated that topical application of 1% TiF4 reduced enamel solubility more efficacious than SnF2, NaF and aluminum phosphate fluoride. He concluded that 1% TiF4 solution would be more protective against dental caries than other substances [17]. Skartveit L et al., also stated that application of TiF4 provides high fluoride content on the root surfaces and can remain at the treated site for a long time [24]. The present study also indicated that use of 1% TiF4 solution may not have major cytotoxic effects on human fibroblast-like cells.
On the other hand, a study by Comar LP et al., comparing the amount of fluoride released from 1.55%, 3.1% and 4% TiF4 varnishes with NaF varnish showed that the 3.1% and 4% TiF4 would release more fluoride content in relation to NaF varnish [28]. This implies that one cannot ignore the better anticaries efficacy of TiF4 in higher concentrations which seemed to be hazardous to vital cells according to the present results.
[Table/Fig-4a] also showed that with increasing the pH level, the percentage of cell viability raised slightly. The pH is a very critical factor for TiF4 solutions. Skartveit L et al., compared the depth of demineralisation and subsequent root surface reaction to native and acidified SnF2 with highly acidic TiF4 [29]. The study showed that the strong bond of titanium-phosphate complex formed at low pH (pH 1) of TiF4 solution made the root surface more resistant to demineralisation in comparison to the other two SnF2 solutions. Anyway, although the result of present study was not incompatible with the previous data, it would suggest higher pH values of TiF4 solutions in order to provide less cytotoxic effects. On the other side, increasing the pH value of TiF4 products is not a practical option till the date. Because major positive effects of TiF4 – like protecting enamel from erosion is mainly due to development of glaze-layer which cannot be formed unless at such low pH value of TiF4 solution. Considering the danger from low pH values, the routine home use of TiF4 products and their application by patients should be limited [30,31].
An old study by Skartviet L et al., comparing TiF4 solutions of 1% and 3.4% after 10 second, 1 minute, 2 minute and 4 minute being in contact with root surfaces, indicated that fluoride content on tooth surfaces were almost the same after treatment with two solutions. The study also showed that prolonging the time periods beyond 1 minute would not increase the Flouride uptake proportionally [24]. This information is somehow in the same way as [Table/Fig-4b&c], representing the influence of interaction of time and other two factors on cell viability, showed. It could be concluded that the ionic strength of solutions and the time of application can be considerably reduced and still benefiting from TiF4’s protective effects, while the safety is ensured.
With the confirmation of the safety of TiF4 application on HGF1-PI 1, the authors would recommend further surveys on the biocompatibility of TiF4 solutions with the optimal operational conditions suggested by this study. It would also be suggested to evaluate the remineralising and preventive ability of TiF4 solution with the operational conditions proved as optimal ones. The RSM could also be utilised to evaluate the changes in the amount of three studied factors on both caries preventive efficacy and cytotoxicity of TiF4 simultaneously.
Limitation
This study was conducted on HGF1-PI 1 as a representative of oral tissues to assess cell reaction to TiF4. Obviously, this isolate cell line could not mimic multiple tissue interactions existing in oral cavity; so the study recommends further evaluation of multiple oral tissue reactions against TiF4. The sensitivity of MTT assay also could be questionable in low amounts of cells per mL and the cell culturing should be implemented cautiously to avoid mistakes.
Conclusion
Our results stated that CCD could be robustly used to determine the optimal level of different elements of operational conditions. It was proved that the three studied factors have significant influence on the cytotoxicity of TiF4 solution. The RSM experiment showed that a level of 2.28 % TiF4 (pH 1.89) in an application period of 1.09 minutes would bring in 86% cell viability amount. The observed level of viable fibroblast-like cells under optimised conditions exhibited 82% vitality, presenting 95.34% agreement between the observed and predicted values which was quite acceptable. Besides, the adequacy of the presented model was also demonstrated. This experiment proved that DOE-based studies could be successfully employed for optimising the operational conditions during TiF4 solution application for reducing the possible toxicity on fibroblast-like cells. Moreover, this method might be applicable for evaluating the cytotoxicity of newly-developed dental materials with some modifications.