An ideal graft material should be osteoconductive (that serves as a scaffold or matrix for bone growth); osteoinductive (that induces bone growth by growth factors or proteins that recruit and stimulate osteoblasts to form bone) and osteogenic (which results in new bone growth directly by osteoblast cells) [1]. Long term stability depends upon the fusion of the involved segment and the rate of fusion depends upon a number of variables including age, primary disease process, presence of osteoporosis, type of surgery performed and the graft material utilised [1,2]. Autologous bone graft is unequivocally the “gold standard” graft material. However, iliac graft is fraught with limitations in the form of limited availability, donor site morbidity and pseudoarthrosis [3-5].
Allogenic bone is far inferior to autograft in terms of osteogenecity, as it only provides a passive scaffold for bone deposition and has other disadvantages such as potential risks of bacterial contamination, possible spread of viral transmission diseases [5,6]. Graft substitutes are the topic of research today and include recombinant human Bone Morphogenetic Proteins (rh-BMP), Platelet Derived Growth Factor (PDGF), platelet concentrate and Demineralised Bone Matrix (DBM) [1,6-9]. Varied osteoconductive scaffolds have been studied, including DBM, hydroxyapatite, and calcium phosphate ceramic materials. They do not have structural strength but possess osteoconductivity and the osteoinductive agents [4,10].
Biologic strategies include use of Bone Morphogenetic Proteins (BMP), cytokines, BMA and gene therapies [1,8,9,11-15]. BMP stimulate pleuripotent mesenchymal cells to differentiate into osteoblasts [1]. Although few reports have documented good efficacy in fusion, relatively high doses of BMP are currently used to produce significant osteoinduction and are associated with many adverse reactions including postoperative radiculitis, heterotopic bone formation, vertebral osteolysis and significant graft subsidence resulting in a loss of graft structural support [16,17].
Mesenchymal Stem Cells (MSCs) which can undergo extensive subcultivation in vitro have been identified and results from a few animal studies are encouraging [18]. However, these cell based therapies are still in their stage of infancy and culture expansion adds cost and extra time. Gene therapy is another new option and although several preclinical studies have demonstrated its efficacy, further work needs to be done in this field [1]. The main drawbacks of these include either the high cost or inconsistent results when used alone or in combination. Also, achieving good fusion in osteoporosis and other bone weakening conditions remains a challenging task for surgeons. Bone marrow aspirate is an attractive option because of its potential biologic value and encouraging results in few animal and human studies [8,9,11-15]. This study aimed to document the usefulness of Bone Marrow Mononuclear Cells (BM-MNC) in bone fusion and to compare the fusion rates of autograft alone versus autograft with BM-MNC.
Materials and Methods
The proposed study was conducted at the Department of Neurosurgery between October 2011 and March 2012, in conjunction with Stem Cell Facility at the All India Institute of Medical Sciences, New Delhi, India in a prospective, non randomised manner after obtaining ethical clearance. A total of 30 patients were included with 15 patients in two groups each [Table/Fig-1]. About 15 patients who underwent instrumentation for Craniovertebral Junction (CVJ) anomalies were enrolled in the study and prospectively analysed. A retrospective age matched cohort of 15 patients previously operated for a CVJ anomaly using autologous iliac crest graft placement was taken as control group. Patients with neoplastic aetiology, blood dyscrasias, chronic smoking, diabetes mellitus, osteoporosis and not giving consent were excluded.
Clinico-radiological characteristics of the two patient groups.
| Parameters | Study group | Control group |
|---|
| Mean age | 30.8 years (14-65 years) | 27.1 years (14-50 years) |
| Male: Female (n)* | 14:1 | 13:2 |
| Pediatric: Adults (n) | 5:10 | 6:9 |
| Mean duration of symptoms | 31 months (Range 4 months to 10 years) | 33.6 months (Range 3 months to 7 years) |
| Diagnosis |
| BI with irreducible AADa (n) | 8 | 9 |
| Reducible AAD (n) | 4 | 4 |
| Odontoidb with atlanto-axial instability (n) | 3 | 2 |
| Associated anomaly |
| Associated bony anomalies (n) | 5 | 7 |
| Associated Chiari I (n) | 4 | 4 |
Abbreviations:*-Number, aAtlanto-axial dislocation, bFracture
For graft harvesting, a 3-4 cm incision was made over the iliac crest around two finger breadths posterior to anterior superior iliac spine. In cases of posterior-only procedures, a similar sized incision was made over the posterior iliac crest. A wedge shaped tricorticate graft of size 4x4 cm2 was harvested followed by aspiration of the bone marrow using 10 mL heparinised syringes attached to a 14G needle. Around 40-50 mL was aspirated which was further processed in the laboratory to yield concentrated BM-MNC (as described in the following section). The sample was made available within 2½ hours duration after assessing for cell viability and BM-MNC count. Simultaneously, incision was made over the desired surgical area and instrumentation was performed. The type of implant used (stainless steel rods versus titanium screws) was dependent on the financial affordability of the patient. The BM-MNC was mixed with ICBG and placed over the decorticated bone around the implant area. Postoperatively, patients were put on a hard cervical collar for a minimum duration of three months and followed up at three and six months after surgery. Radiographs (static±dynamic) ± Computed Tomography (CT) were performed at follow-up periods to assess bone fusion. All radiographs were analysed by the study supervisor (SSK), in conjunction with an independent radiologist.
Based on the pattern of bone fusion achieved, we randomly divided it into three patterns: absent - if no bone fusion was visible; partial/incomplete bony - if bony trabeculae were visible but a solid mass had not yet formed; dense/complete bony fusion - if a solid bony mass was seen over the fused site.
Also, the donor site complications were noted. The length of donor site incision was documented in every case of study group and assessed in relation to chronic pain which was defined as pain persisting for more than one month after surgery. Medical records of control group were retrieved and reviewed with respect to clinico-radiological features, surgical interventions, complications and fusion (at three and six months).
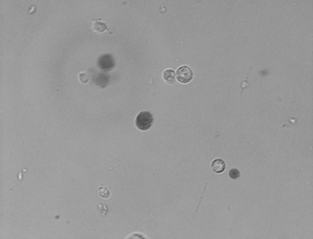
In laboratory, the Mononuclear Cells (MNC) were separated by Ficoll density separation method [19]. The cell count and viability was tested. Cell count was done using a Neubauer-type haemacytometer counting chamber and a contrast phase microscope using manual cell counts. The total number of cells were determined using the following calculation: cells per mL = the number of cells counted in 16 small squares × the dilution factor × 104. Cell viability was tested using tryptan blue dye exclusion assay. This is based on the pump mechanism present in active live cells which pumps the dye out of the cell, thus rendering live cells as clear and dead cells as dark coloured [Table/Fig-2]. Percentage of live cells was calculated using the following equations: viability (%) = total no. of white cells × 100/ (total no. of white and blue cells).
Bone marrow MNCs mixed with tryptan blue dye for viability test. Live cells are seen as clear circles while dead cells are seen as dark circles (4X).
Statistical Analysis
Assuming the fusion rate of control group to be 70% and study group as hypothesised to be >95%; with a statistical power of 80% and α of 5%, minimum of 12 patients were needed for the study. We included 15 patients in each group, more than the required statistical numbers. All statistical analyses were performed using Stata software (version 9.0). Qualitative data such as fusion rates (with respect to different parameters) were analysed by Fisher’s exact test. A p-value of <0.05 was considered significant.
Results
The demographic and radiological data of both the groups have been summarised in [Table/Fig-1].
Clinical Presentation
Progressive spastic weakness with myelopathy was the most common presenting feature noted in both groups of patients. Other symptoms included neck pain, numbness, urinary dysfunction, parasthesia, lower cranial nerve palsy, neck clicks and restriction of neck. Surgical details of both the groups have been summarised in [Table/Fig-3].
Surgical procedures performed in the groups.
| Surgical details | Study group n (%) | Control group n (%) |
|---|
| Type of procedure |
| TOOa +PFb | 8 (53%) | 9 (66%) |
| PF | 7 (47%) | 6 (34%) |
| FMDc | 3 (20%) | 4 (27%) |
| Type of fixation |
| Titanium wire | 2 (13%) | 1 (7%) |
| Contoured steel rod and wire | 1 (7%) | 2 (13%) |
| Titanium screw and rod | 12 (80%) | 12 (80%) |
| Type of posterior fixation |
| Occiput to C-3,4/C-4,5 LMd SRFe | 5/2 | 7/2 |
| Occiput to C2 TLf SRF | 4 | 1 |
| C1-C2 TASg (with Sonntag fusion) | 1 | 1 |
| C1 LM, C2 pedicle screws | 0 | 1 |
| Gallie’s/Brook’s procedure | 2 | 1 |
| Contoured steel rod and wire | 1 | 2 |
Abbreviations: a Trans-oral odontoidectomy; b Posterior fixation; c Foramen magnum decompression; d Lateral mass; e Screw and rod fixation; f Translaminar; g Trans-articular screws
BM-MNC details: The average time duration for procurement of BM-MNC was 98 min (range 70-130 min). An average of 40.6 million cells (range 10-67 million) were utilised. Volume of the cell concentrate utilised ranged from 1 to 3 mL with a mean volume of 1.6 mL. In all cases, viability of cells used were ≥98% (mean-98.2%; median-98%; range-98-100%). Mean length of graft site skin incision at the donor site was 3.7 cm (3-5 cm).
Complications were categorised as intraoperative, wound related and infectious as summarised in [Table/Fig-4].
Complications noted in the patient groups.
| Type of complications | Study group (n) | Control group (n) |
|---|
| Intraoperative CSF leak | 1 | 2 |
| Wound related |
| CSF leak from wound | 1 | 1 |
| Wound infection | 1 | 1 |
| Re-exploration of oral wound | 0 | 1 |
| Skin breakdown at implant site | 1 | 0 |
| Infectious |
| Pneumonia | 1 | 0 |
| Meningitis | 0 | 1 |
| Graft site related |
| Chronic pain | 2 | 3 |
| Seroma | 0 | 1 |
Abbreviations: CSF- cerebrospinal fluid
Follow up and outcome: The mean postoperative hospital stay was 10.9 days in the study group and 10.3 days in control group. In the study group, one patient was lost to follow up after three months. Rest of the 14 patients were followed up regularly at three and six months with cervical X-rays being done at each visit. Similar follow up data was obtained for control group through medical case records. With respect to neurological outcome, in the study group, one patient had transient worsening of motor power. In the control group, one patient had delayed worsening of deficits which has remained stable since then. In all other patients, there was symptomatic improvement in the postoperative period.
Bone Fusion
On comparing the fusion rates between the two groups, we found a higher proportion of bone fusion in study group [Table/Fig-5,6] at both three and six months of follow up compared to control group [Table/Fig-7], which was not statistically significant [Table/Fig-8a,b]. About 40 million was the mean and median cell counts used and based on this we classified into two subgroups- <40 million (nine patients) and >40 million (six patients). Also, two different BM-MNC volumes were used in the study group- <1.5 mL (10 patients) and >1.5 mL (five patients) and this division was also based on its mean value of 1.5 mL. On analysis of fusion rates in relation to these two parameters, no significant difference could be obtained [Table/Fig-9a,b].
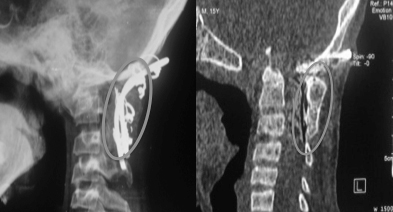
Postoperative radiograph of the Craniovertebral (CV) junction showing good bony fusion (grey circle) at three months after surgery with contoured steel rod and wire fixation for a case of irreducible atlanto-axial dislocation in a patient of study group (left). Computed tomography of CV junction showing dense bone formation (grey circle) at six months follow up in the same patient (right).
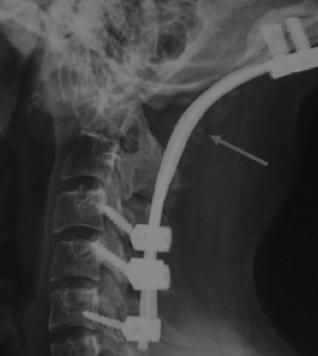
Postoperative X-ray CV junction showing incomplete bony fusion (arrow) at six months after surgery in a case of study group who underwent occipito-cervical screw and rod fusion.
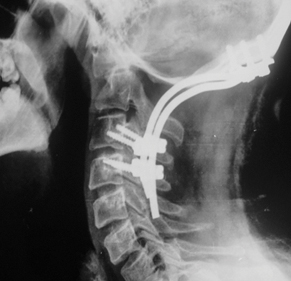
Postoperative X-ray CV junction-lateral view showing absent fusion at six months after surgery in a case of control group who underwent occipito-cervical screw and rod fusion.
Patterns of bone fusion noted in both the groups.
| Type of bone fusion | Study group (n) | Control group (n) |
|---|
| 3 months FU | 6 months FU | 3 months FU | 6 months FU |
|---|
| Dense bony | 2 | 7 | 0 | 3 |
| Incomplete bony | 7 | 4 | 3 | 4 |
| Absent | 6 | 3 | 12 | 8 |
Abbreviations: FU- Follow Up
Overall fusion rates compared between both groups at three and six months follow up.
| Study group n (%) | Control group n (%) | p-value |
|---|
| Fusion at 3 months | 9/15 (60%) | 3/15 (20%) | 0.06 |
| Fusion at 6 months | 11/14 (78%) | 7/15 (47%) | 0.26 |
Fusion rates in study group in relation to BM-MNC count.
| <40 million | >40 million | p-value |
|---|
| Overall fusion (n) (%) | 7/9 (78%) | 4/6 (67%) | 1.00 |
Fusion rates in the study group in relation to volume of BM-MNC.
| <1.5 mL | >1.5 mL | p-value |
|---|
| Overall fusion (n) (%) | 7/10 (70%) | 4/5 (80%) | 1.00 |
BM-MNC- Bone Marrow Mononuclear Cell
As already mentioned, chronic pain was separately assessed in study group. We arbitrarily formed two subgroups based on the average length of graft site skin incision- <3.5 cm (n=9) and >3.5 cm (n=6). None had chronic pain in the former category while two patients had chronic pain in the latter category. The length of skin incision had no relation to bone fusion.
Discussion
Among autografts, iliac crest autograft typically is cortico-cancellous, providing both structural support, possessing all the three properties (osteoinduction, osteoconduction and osteogenicity) and hence considered as the “gold standard” for bone grafting in spinal surgery [1,20]. However, limitations include limited bioavailability, associated donor site morbidity and pseudoarthrosis [3-5]. These problems have led surgeons to devise new biological strategies and alternative substitutes for autograft. These include allograft, DBM, bioactive strategies such as BMP and cytokines, cellular approaches such as unfractionated fresh bone marrow and marrow derived-culture expanded Mesenchymal Stem Cells (MSC), and gene therapy [1,4,17]. Although newer graft materials avoid the complications of iliac crest donor site morbidity, equivalent or better rates of fusion need to be demonstrated and hence, the ideal graft still remains an enigma.
Burwell RG described the osteogenic potential of bone marrow in 1964 [21]. It induces bone formation by proliferation and differentiation of osteoprogenitor cells contained in marrow cavities. In a healthy young patient, for every 50,000 nucleated bone marrow cells, one cell is an osteoprogenitor cell [21]. Successful use of bone marrow aspirate has been described alone or in combination with other bone graft substitutes [1,8,9,11-14,22,23]. BMA contains a variety of cells and the filtered concentrate devoid of platelets, RBC’s and plasma possesses mononuclear cells which are not related to stem cells. The principal advantages of using this approach are that the procedure is simple and relatively inexpensive. It is said that the dilution effect of bone marrow aspiration can be reduced by limiting the volume of aspiration from a given needle site to 2 cc or less or by means of centrifugation techniques [24]. Studies by Bansal S et al., Khoueir P et al., Ajiboye RM et al., and Niu CC et al., have documented good fusion rates using BMA combined with synthetic graft substitutes while few studies failed to show superior results with BMA as compared to ICBG alone [8,9,11,12]. In our study, aspiration was done at multiple sites to negate the dilution effect as mentioned above in addition to centrifuging the sample. The iliac graft was first harvested, followed by marrow aspiration thus making sure the graft harvested also contained sufficient amount of osteoprogenitor cells. However, in contrary to earlier studies of Bansal S et al., and Khoueir P et al., we utilised a concentrated marrow aspirate which was processed in laboratory, in combination with autograft for enhancing fusion [8,9]. Also, this entire laboratory procedure took on an average 2½ hours and the required instrumentation was performed during this processing time. Thus, there was no extra time wasted in comparison to standard techniques.
For assessment of bone fusion, the gold standard remains direct surgical assessment [25]. However, this is not practical and hence fusion was assessed using non-invasive techniques. Dynamic radiographs are better than static radiographs as documented in literature. A few authors have found CT to been more sensitive in detection of abnormalities than both static and dynamic radiographs [26]. In our study, bone fusion was assessed by static and dynamic radiographs ± CT. Both at three and six months, there were higher fusion rates noted in study group which was not statistically significant because of the smaller cohort. At three months, the overall fusion rate was 60% and 20%, favouring the study group, while it was 78% and 47% at six months for study and control groups respectively (p-values 0.06 and 0.26 respectively). Also, authors found no statistical significance in bone fusion with either the number of cells used or the concentration of the aspirate (p-value 1.00). Again, this might be due to the very small cohort sample included in the study group.
In cases with osteoporosis or other such bone weakening conditions, achieving bone fusion is a challenging task due to inherent poor quality of bone. A number of options have been described for such cases in literature. These include use of augmented screws (with poly methyl methacrylate bone cement), zolendronic acid, teriparatide, expandable screws, double screws and others [27-32]. However, all these alternatives come with an extra cost to be borne by the patient, which on many occasions might not be feasible in developing countries like ours. The use of concentrated marrow aspirate with autograft might prove beneficial in such scenarios, achieving satisfactory bone fusion and eventually the outcome.
Merits of the study: a) All the studies available in literature on BMA are either animal studies or have utilised unconcentrated bone marrow or graft substitutes along with BMA. This is first of a kind study which utilised concentrated BM-MNC (processed systematically) combined with iliac graft to evaluate its efficacy in bone fusion; b) Also, results with standard procedures (without BM-MNC) were compared, albeit in retrospective manner. We found a higher proportion of bony fusion in the study group as compared to control group both at three and six months follow up; c) It supports the results of earlier reports that length of graft site incision correlates with intensity and chronicity of pain [33].
Limitation
The limiting factors in our study were: a) cohort sample size was small in both groups, so as to comment with confidence on the superiority of this technique over the standard one; b) control group was retrospectively analysed.
This study was a pilot study using a small cohort with CVJ anomalies. Because of higher fusion rates obtained with the combination technique, authors propose to include it in the spine surgeons’ armamentarium as an alternative technique for patients with osteoporosis and other bone weakening conditions which might well augment the fusion rate, thus avoiding use of graft substitutes and its inherent extra cost, especially in developing countries like ours. However, as of now, further prospective studies are needed in larger proportion to confirm these results so as to be applied on a regular basis in all spinal fusion cases and also to optimise the volume and number of mononuclear cells required.
Conclusion
BM-MNC with iliac graft lead to a higher proportion of bony fusion and seem to be effective in all varieties of fixation procedures. Smaller donor site incision correlates with lesser incidence of chronic pain. Patients with osteoporosis might benefit with this combination technique. Although, it appears to be a promising option for enhancement of bone fusion at an economical cost with no extra procedural time, further prospective studies in larger numbers are needed to confirm these findings.
Abbreviations:*-Number, aAtlanto-axial dislocation, bFracture