Tracheal Amyloidosis Presenting as Life Threatening Airway Obstruction
Alkesh Kumar Khurana1, Vikas Gupta2, Abhishek Goyal3, Ganakalyan Behera4, Garima Goel5
1 Associate Professor, Department of Pulmonary Medicine, All India Institute of Medical Sciences, Bhopal, Madhya Pradesh, India.
2 Associate Professor, Department of ENT and Head and Neck Surgery, All India Institute of Medical Sciences, Bhopal, Madhya Pradesh, India.
3 Associate Professor, Department of Pulmonary Medicine, All India Institute of Medical Sciences, Bhopal, Madhya Pradesh, India.
4 Ex Senior Resident, Department of ENT and Head and Neck Surgery, All India Institute of Medical Sciences, Bhopal, Madhya Pradesh, India.
5 Associate Professor, Department of Pathology and Lab Medicine, All India Institute of Medical Sciences, Bhopal, Madhya Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Alkesh Kumar Khurana, C-100, Emerald Park City, Bagsewania, Bhopal-462043, Madhya Pradesh, India.
E-mail: lungcancer@rediffmail.com
We hereby present a case of a 62-year-old man who presented with tracheostomy in situ for the last two years and unexplained etiology for the life-threatening airway compromise. Thorough work up revealed tracheal amyloidosis as the cause for airway obstruction. This case describes not only the uncommon presentation of the disease but also the isolated nature of deposition of amyloid.
Amyloid, Subglottis, Tracheostomy
Case Report
A 62-year-old male presented to the pulmonary medicine Out Patient Department (OPD) with a tracheostomy in situ for last two years. He had a history of insidious onset progressively worsening dyspnea, two and half years back. Two years back he had presented to another hospital with life-threatening dyspnea/airway obstruction and tracheostomy was done as an emergency resuscitative measure. Tracheostomy had remained in situ and all attempts to decanulate had failed. He was now evaluated in detail for all possible causes of airway obstruction at our centre. There was no significant past history except that the patient was a known Type II diabetic on oral hypoglycemics.
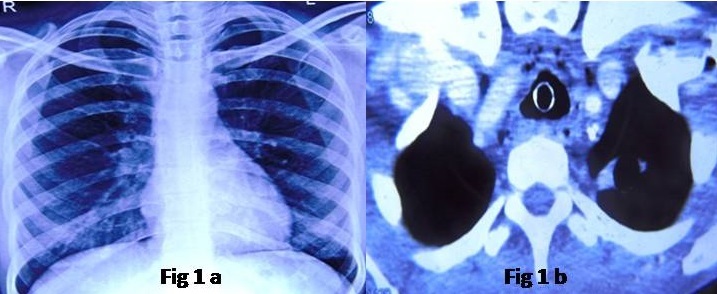
His chest X-ray was repeatedly normal [Table/Fig-1a]. Computed Tomography (CT) revealed generalised soft tissue thickening in supraglottis and glottis region with circumferential tracheal wall thickening [Table/Fig-1b] and no chest parenchymal abnormality. There was no mediastinal lymph node enlargement. Routine investigations namely, complete and Differential Blood Count (Hb=11.5 gm/dL, TLC=10.94×109/L, Platelets=205×109/L, DLC=N70L20M6E3Ba1), serum electrolytes and liver function tests were within normal limits. The renal function tests revealed a high blood urea (55 mg/dL) and high serum creatinine levels (1.35 mg/dL). His Plasma glucose levels were above 300 mg/dL and HbA1c was 10%. The urine examination revealed albuminuria (1087.5 mg/L) and urine albumin/creatinine ratio of 1806.47 μg/mg. Anti-diabetic treatment was stepped-up immediately and simultaneously he was worked up to find out the reason for compromised airway. Flexible bronchoscopy was done via tracheostomy site which showed no significant abnormality in the tracheobronchial tree except for circumferential narrowing in the subglottic area. When attempted via the nasal route, there was diffuse edematous swelling in the glottis area and the scope could not be negotiated through the vocal cords. Also, he was investigated for Wegener’s granulomatosis but Anti-neutrophilic cytoplasmic antibodies (both c-ANCA and p-ANCA) were negative. The patient was further evaluated under Department of ENT-Head and Neck Surgery with direct laryngoscopy and tracheoscopy under general anaesthesia. There was generalised mucosal thickening in the supraglottis, glottis and suprastomal trachea. Though, there was compromise of the airway at glottis and subglottis region also but the tracheal component was most significant. There was soft, circumferential narrowing of the trachea right from the subglottis upto tracheostomy site. Histopathological examination of endoscopic biopsies from glottis and tracheal tissues were inconclusive.
a) Grossly normal chest radiograph (PA view); b) CT thorax showing diffuse thickening of the tracheal wall with tracheostomy tube in situ.
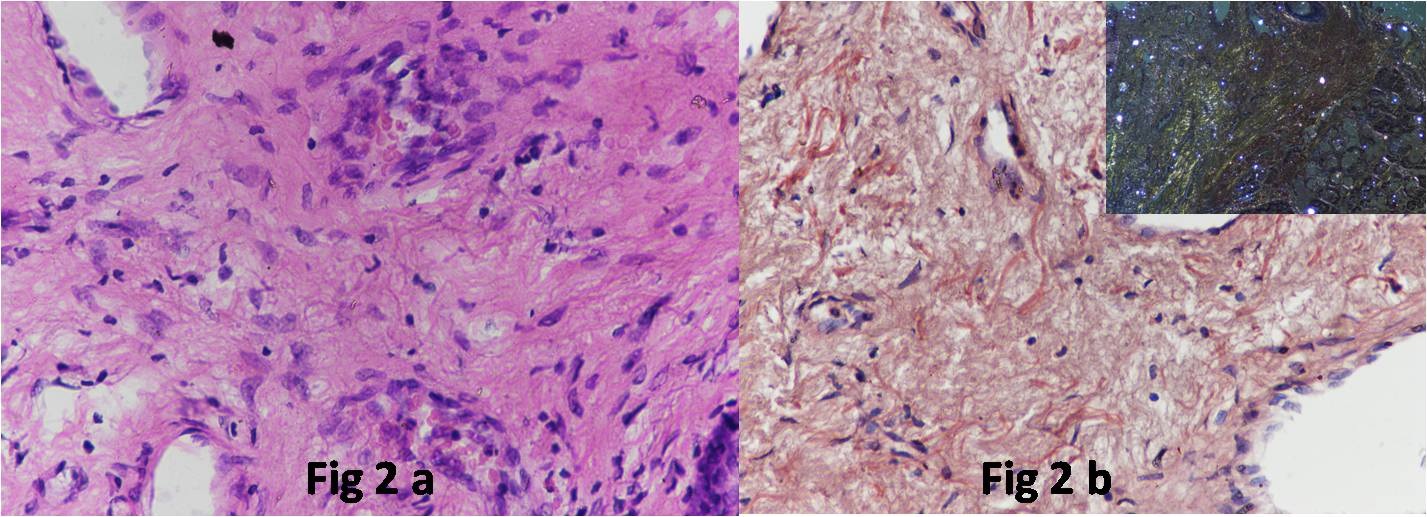
Next, an open biopsy was taken from the suprastomal trachea including tracheal cartilage and the medial soft tissue under local anaesthesia. The biopsy revealed extracellular, hyaline, eosinophilic, amorphous deposits on routine Haematoxylin and Eosin (H&E) sections [Table/Fig-2a]. On Congo red stain, this substance showed congophilia and on polarization a characteristic apple green birefringence confirming the substance to be amyloid [Table/Fig-2b]. After the biopsy diagnosis, the patient underwent abdominal fat pad and kidney biopsy to look for systemic amyloid deposits. There was no evidence of deposition of amyloid in both the tissues. There was no cardiac manifestation of amyloidosis. The serum protein electrophoresis did not reveal any M band. The patient did not give consent for a bone marrow biopsy.
a) The biopsy revealed extracellular, hyaline, eosinophilic, amorphous deposits on routine H&E sections. (H&E; 10X); b) On Congo red stain these deposits showed congophilia. (Congo red; 10X) Inset shows characteristic apple green birefringence on polarizing microscopy.
The indexed case is reported in view of its rare type of clinical presentation and isolated organ of amyloid deposition being trachea.
Discussion
Amyloidosis is a group of disorders characterised by the extracellular deposits showing specific histochemical and tinctorial properties. All amyloid deposits look similar morphologically and physically. However, they are different chemically; more than 20 types of protein can aggregate and form fibrils with the appearance of amyloid. The three most common forms of amyloid are Amyloid Light (AL) chain type; Amyloid Associated (AA) type; and β-Amyloid protein (Aβ) [1]. Amyloidosis may be systemic i.e. involving different organs or can be localized to a specific organ. Pulmonary or tracheobronchial amyloidosis is usually a part of systemic amyloidosis but in the indexed case there was only tracheal amyloidosis. Abdominal fat pad and kidney biopsy were negative for amyloid although patient refused bone marrow aspiration and biopsy. It has been reported that almost 90% of patients with systemic amyloidosis have pulmonary involvement in an autopsy series [2]. On the contrary, approximately 60% patients of pulmonary amyloidosis have systemic disease [3]. Isolated amyloidosis of the respiratory tract can present as (1) infiltrative parenchymal nodules (2) nodular parenchymal amyloid (3) tracheobronchial amyloid and (4) pseudo tumour tracheobronchial amyloid which has the discrete appearance of a tumour [1]. Although, nearly 200 cases of primary isolated tracheobronchial amyloid have been reported, isolated diffuse tracheal amyloidosis as in this case, is much rarer [1]. Clinical presentation can vary from being asymptomatic to be having cough, hemoptysis or in extreme cases stridor, such as this one. History of recurrent pneumonias or dyspnea suggest pulmonary involvement [4]. At bronchoscopy, the submucosal plaques of amyloid have been described as shiny and pale ridges because of stretching of surface epithelium [5]. The patient here had no bronchoscopic findings (and normal lung parenchyma on CT thorax) emphasising that the amyloid deposition was localised to within the tracheal wall. This patient was initially suspected to be having a malignant disease at the presentation two years back. Non-progression of the disease severity for two years strengthened the possibility of a benign disease when the patient presented to us and henceforth a possibility of amyloidosis was kept in the differential diagnosis.
Possible therapeutic options in such cases include bronchoscope guided stent placement, tracheostomy to regain airway patency (as done in this case) or resection of short tracheal segments in cases of localised pseudo tumour disease [6]. However, surgical procedures have a couple of drawbacks here. One is the risk of bleeding because of friable nature of amyloid and the other being possibility of stricture formation, especially when the patient has to undergo multiple procedures [7]. For this reason, radiotherapy has also been used in such cases with an aim of disease regression and possible reduction in need of surgery [1]. Chemotherapy with colchicine and steroids has been reported with some effect in systemic amyloidosis but not found favourable for localised tracheobronchial disease [8]. This patient refused further surgical treatment and is on follow up with regular tracheostomy care.
Conclusion
Tracheobronchial amyloidosis is a rare disease and isolated tracheal amyloidosis is even rarer. Diagnosis is dependent on tissue biopsy with Congo red stain demonstrating “apple green” birefringence. Surgical resection and/or radiotherapy have been performed with some success. This entity should be kept as a differential diagnosis in a patient who present with airway narrowing.
[1]. Numbere NK, Grayez J, A rare cause of stridor: Isolated tracheal amyloidosisCan Respir J 2014 21(5):273-75. [Google Scholar]
[2]. Smith RR, Hutchins GM, Moore GW, Humphrey RL, Type and distribution of pulmonary, parenchymal and vascular amyloid. Corelation with cardiac amyloidosisAm J Med 1979 66(1):96-104. [Google Scholar]
[3]. Utz JP, Swensen SJ, Gertz MA, Pulmonary amyloidosis: The Mayo Clinic experience from 1980 to 1993Ann Intern Med 1996 124(4):407-413. [Google Scholar]
[4]. Aggarwal AN, Gupta D, Joshi K, Jindal SK, Tracheobronchial amyloidosis: A report of two casesIndian J Chest Dis Allied Sci 2000 42(2):115-118. [Google Scholar]
[5]. Thompson PK, Citron KM, Amyloid and the lower respiratory tractThorax 1983 38(2):84-87. [Google Scholar]
[6]. Lang EE, Phelan E, Rowley H, Tracheal amyloidosis-an unusual cause of stridorEar Nose Throat J 2009 88(5):E27 [Google Scholar]
[7]. Alloubi I, Thumerel M, Begueret H, Baste JM, Velly JF, Jougon J, Outcomes after bronchoscopic procedures for primary tracheobronchial amyloidosis. Retrospective study of 6 casesPul Med 2012 2012:352719 [Google Scholar]
[8]. Birkeland AC, McHugh JB, Spector ME, Tracheobronchial amyloidosis: A case report and review of literatureJ Case Rep Med 2014 3(pii):235859 [Google Scholar]