Introduction
Diabetes is a non-communicable metabolic disorder with an estimated prevalence of 382 million worldwide and 65 million in India, which is predicted to increase to 100 million by 2030 [1]. Diabetes is well known to cause both vascular (micro and macrovascular) and non vascular complications. Neuropathy is one of the most frequently encountered microvascular complications and along with peripheral vascular disease; it is one of the leading causes of non-traumatic lower limb amputation [2].
The prevalence of diabetic neuropathy in the Indian population ranges from 19.1% [3] to 29.2% [4]. Distal symmetrical neuropathy is the most common form of diabetic neuropathy, and its prevalence has been reported to be as high as 50 to 75% among type 2 DM patients [5,6]. The gold standard of diagnosis of peripheral neuropathy has been the NCS. However, it is cumbersome and expensive and not widely available [7]. Therefore, a clinical scoring system which can be easily performed and that correlates well with NCS, is needed in resource poor settings like India. Several scoring systems have been introduced for diagnosis and classification of DPN, and include the TCNS and its modified score (mTCNS), Michigan Neuropathy Screening Instrument (MNSI), Neuropathy Impairment Score (NIS) among others [7,8].
In India, the commonly used tests are the Semmes-Weinstein Monofilament test (SWM), Vibration Perception Test (VPT), Neuropathy Symptom Score (NSS) and Neuropathy Disability Score (NDS) [9-11]. The sensitivity and specificity of these scores have been compared by Mythili A et al., and the VPT was found to have the best sensitivity of 86% and specificity of 76%. The TCNS, introduced by Bril V and Perkins B, has been evaluated in several studies from Canada and the United States, and has been found to have a significant correlation with sural nerve myelinated fiber density in patients with diabetic neuropathy [12]. However, this score has not been validated in the Indian population. The present study evaluated the applicability of TCNS in diagnosing DPN in South Indian population. The primary aim of the present study was to evaluate the applicability of TCNS in South Indian diabetic patients with peripheral neuropathy and to establish its correlation with NCS. The secondary objectives included correlation of the severity of DPN with the duration of diabetes, diabetic control (as assessed by HbA1C) and with other microvascular complications.
Materials and Methods
Study Design
This was a prospective observational study carried out in the medicine wards of a tertiary care university teaching hospital in semi-urban Southern India over a period of 12 months (June 2015 to May 2016). The study included all patients above the age of 18 years with type 1 or type 2 Diabetes with symptoms suggestive of peripheral neuropathy. Patients with neuropathy due to causes other than diabetes and those who refused informed consent were excluded from the study. Sample size was calculated based on a 19.1% prevalence of neuropathy in diabetes in South India [3], allowing for an error of 10%, and thereby a total of 50 patients were recruited.
Data Collection
After obtaining approval from the Institutional Ethical Committee, patients were recruited in the study based on the inclusion criteria. Informed consent was obtained and the patients were subjected to history and physical examination, including assessment of the TCNS score. They were then evaluated with NCS using RMS EMG EP Mark2 recorder, medicare systems and evaluation of motor function of the median, ulnar, peroneal, and tibial nerves, and sensory function of median, ulnar, radial, and sural nerves were performed. Velocities were documented in meters per second, motor amplitudes in millivolts, and sensory amplitudes in microvolts. A pre-structured proforma was used to record demographic details of the patients. The patient’s clinical profile including age, gender, and duration of diabetes, HbA1c and associated microvascular complications were documented.
The individual patient’s TCNS score was documented out of a total of 19. Severity of neuropathy was classified based on the score as: no neuropathy (0 to 5), mild neuropathy (6 to 8), moderate (9 to 11) and severe diabetic neuropathy (12 to 19) [Table/Fig-1].
Toronto Clinical Neuropathy Scoring System (TCNS) [13].
| Symptom scores | Reflex scores | Sensory test scores |
|---|
| Foot | Knee reflexes | Pinprick |
| Pain | Ankle reflexes | Temperature |
| Numbness | | Light touch |
| Tingling | | Vibration |
| Weakness | | Position |
| Ataxia | | |
| Upper-limb symptoms | | |
Sensory testing was performed on the first toe. Symptom scores: present = 1; absent = 0. Reflex scores: absent = 2; reduced = 1, normal = 0. Sensory test score: abnormal = 1. normal = 0. Total scores range from normal = 0 to maximum of 19.
Data Analysis
Continuous variables were assessed for the normality using Shapiro – Wilk’s test. If the variables were normally distributed they were expressed as mean±standard deviation, otherwise median (interquartile range). Categorical variables were expressed either as percentage or proportions. Comparison of normally distributed continuous variables was done by independent sample t-test, abnormally distributed continuous variables by Mann-Whitney U test, and categorical variables by either Chi-square test or Fisher’s exact test based on the number of observations. Data analysis and validation was carried out by SPSS v.11.0. All p-values less than 0.05 were considered statistically significant.
Results
In our study, the mean age of the study population was 59.9 years (±12.89). The mean duration of diabetes in the study population was 8.40 (±6.09) years. Most of the patients had poorly controlled diabetes with a mean HbA1C of 10.2% (SD± 2.10%). There were four patients (8%) with Type 1 DM. The baseline characteristics of the patients are represented in [Table/Fig-2].
Baseline characteristics.
| Clinical Parameter | Total Number of patients N=50 n (%) | Mean |
|---|
| Age (in years) | < 40 | 4 (8) | 59.9±12.89 |
| 41-50 | 10 (20) |
| 51-60 | 13 (26) |
| 61-70 | 13 (26) |
| >70 | 10 (20) |
| Gender | Male | 29 (58) | --- |
| Female | 21 (42) |
| HbA1c (%) | | | 10.2±2.10 |
| Duration of diabetes (in years) | <5 | 21 (42) | 8.40 (±6.09) |
| 5-10 | 18 (36) |
| >10 | 11 (22) |
| Associated microvascular complications |
| Retinopathy | No retinopathy | 20 (40) | --- |
| NPDR | 24 (48) |
| PDR | 6 (12) |
| Nephropathy | No nephropathy | 23 (46) | --- |
| Microalbuminuria | 20 (40) |
| Macroalbuminuria | 7 (14) |
Evaluating for the primary objective, the applicability of TCNS and its correlation with NCS was studied. Out of the total of 50 patients, 49 (98%) were found to have a TCNS score of 6 or more, clinically indicating the presence of neuropathy. The distribution of the signs and symptoms is depicted in [Table/Fig-3]. It was noted that all 50 patients (100%) had foot pain, numbness and tingling. Upper limb symptoms were observed in 41 (82%) patients. On sensory testing, pin prick was diminished in 48 (96%) whereas vibration sense was reduced only in 12(24%). Ankle reflex was reduced in 20 patients (40%) and absent in 30 patients (60%).
Distribution of symptoms and signs of peripheral neuropathy.
| Symptoms | Number of patients (%) | Sensory testing | Number of patients (%) |
|---|
| Foot pain | 50 (100) | Pinprick | 48 (96) |
| Foot numbness | 50 (100) | Temperature | 18 (36) |
| Foot tingling | 50 (100) | Light Touch | 42 (84) |
| Foot weakness | 14 (28) | Vibration Sensation | 12 (24) |
| Ataxia | 12 (24) | Joint Position | 19 (38) |
| Upper limb symptoms | 41 (82) | | |
| Reflex Scores |
| Knee reflexReducedAbsent | 20 (40)13 (26) | Ankle reflexReducedAbsent | 20 (40)30 (60) |
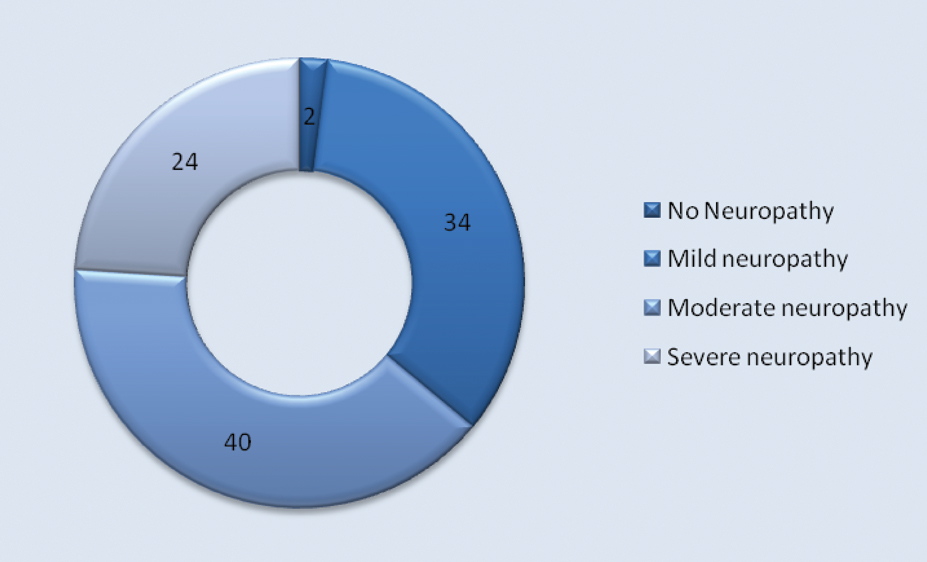
The severity of the neuropathy was graded based on the TCNS. Twelve (24%) of patients were diagnosed to have severe DPN [Table/Fig-4] while 20 (40%) and 17 (34%) had moderate and mild neuropathy respectively. Forty eight out of 49 patients who had clinical neuropathy by TCNS were subsequently confirmed by NCS to have peripheral neuropathy (97.9%). One patient who did not have neuropathy by TCNS was found to have neuropathy according to NCS.
Distribution of severity of neuropathy as assessed by TCNS in percentage.
On analysis of the secondary objectives, the duration of diabetes correlated well with the severity of diabetic neuropathy. Patients who had diabetes for more than five years had either moderate or severe diabetic neuropathy as compared to those with lesser duration of diabetes (<5 years) with p<0.001. However, the severity of neuropathy was not found to have significant association with the degree of glycaemic control (as reflected by HbA1c) p=0.135 [Table/Fig-5].
Correlation of severity of neuropathy with risk factors and other microvascular complications.
| Parameter | No DN(%) | MildDN(%) | Moderate DN(%) | Severe DN(%) | p-value |
|---|
| Duration of diabetes (years) | ≤5 | 1 (4.8) | 12 (57.1) | 7 (33.3) | 1 (4.8) | 0.015 |
| 6-10 | 0 | 4 (22.2) | 6 (33.3) | 8 (44.4) |
| >11 | 0 | 1 (9.1) | 7 (63.6) | 3 (27.3) |
| HbA1c (%) | <9 | 1 (7.7) | 6 (46.2) | 5 (38.5) | 1 (7.7) | 0.135 |
| >9 | 0 | 11 (29.7) | 15 (40.5) | 11 (29.7) |
| Diabetic retinopathy | No retinopathy 20 (40%) | 1(5) | 14 (70) | 4 (20) | 1 (5) | <0.001 |
| NPDR 24 (48%) | 0 | 2 (8.3) | 15 (62.5) | 7 (29.2) |
| PDR 6(12%) | 0 | 1 (16.7) | 1 (16.7) | 4 (66.7) |
| Diabetic nephropathy | No nephropathy 23 (46%) | 1 (4.3) | 15 (65.2) | 6 (26.6) | 1 (4.3) | <0.001 |
| Micro-albuminuria 20 (40%) | 0 | 1 (5) | 13 (65) | 6 (30) |
| Macro-albuminuria 7 (14%) | 0 | 1 (14.3) | 1 (14.3) | 5 (71.4) |
DN: Diabetic Neuropathy; NPDR: Non Proliferative Diabetic Retinopathy; PDR: Proliferative Diabetic Retinopathy.
The severity of other microvascular complications such as retinopathy and nephropathy was compared against the severity of diabetic neuropathy. Among patients with mild diabetic neuropathy only 3 (17.6%) had any evidence of retinopathy, whereas in those patients with moderate to severe neuropathy 22 (68.75%) had Non Proliferative Diabetic Retinopathy (NPDR) and 5 (15.62%) had Proliferative Diabetic Retinopathy (PDR) which was statistically significant p<0.001 [Table/Fig-5]. Similarly, while assessing the presence of co-existing nephropathy in patients with diabetic neuropathy, only 2 (11.7%) of the patients with mild diabetic neuropathy had evidence of diabetic nephropathy as compared to patients with moderate to severe diabetic neuropathy, 25(78%) of whom had nephropathy (p<0.001).
Discussion
Diabetes is one of the most prevalent metabolic disorders all over the world causing significant morbidity and mortality. A few studies have been done so far on the relationship between TCNS and diabetic neuropathy. Among these, important studies include those done by Bril V et al., [12-14] and Bostani A and Homayuonfar H [15], It is appropriate to compare the present study with their findings.
In the present study mean age was a 59.9±12.89 year which was similar to the above mentioned studies. Out of the total of 50 patients 29 (58%) were males and 21 (42%) females. This is in contrast to Bostani A and Homayuonfar H, in whose study 20% were males and 80% were females [15]. However, other authors such as Bril V et al., have showed distribution among males varying from 61-65% and for females from 35-38%.
When mean duration of diabetes was studied, it was found to be 8.40±6.09 years in this study. This is significantly different from other studies where mean duration of diabetes was more than 11 years. Although, this indicates that patients in current study had relatively shorter duration of diabetes, nevertheless, the duration of diabetes had a statistically significant correlation with the severity of diabetic neuropathy. Similar findings were reported by Ashok S et al., and Gill HK et al., in their research on risk factors associated with diabetic neuropathy [3,4].
The next variable analyzed in the study was the degree of sugar control as determined by mean HbA1C values. The study population had a poorer control of diabetes (mean HbA1C 10.2±2.10%) as opposed to patients of Bril V et al., (mean HbA1C of 8.5±1.7%). But interestingly in this study, the severity of neuropathy had no correlation with their HbA1C. A similar lack of correlation between HbA1C and diabetic neuropathy has been reported by Gill HK et al., who proposed that any level of elevated glucose beyond a particular threshold will predispose to neuropathy, and not necessarily a linear correlation [4,16].
With regards to severity of neuropathy, as assessed by TCNS, out of 50 patients who were included in this study, (1) 2% had no diabetic neuropathy, (17) 34% mild, (20) 40% moderate and (12) 24% had severe diabetic neuropathy. Similar study done by Bril V et al., with 65 patients, 12.3%, 21.5%, 27.7% and 38.5% had no neuropathy, mild neuropathy, moderate neuropathy and severe diabetic neuropathy respectively.
Since neuropathy is known to be associated with other microvascular complications such as retinopathy and nephropathy, the current study also analyzed the presence of other co-existing microvacular manifestations. In our study, it was found that among neuropathy patients, 60% had retinopathy and 54% had nephropathy. Comparing this data with a few other studies from India, Bansal D et al., reported a prevalence of 41.8% retinopathy and 20.9% nephropathy among patients with diabetic neuropathy [17]. In the CURES-55 study from Chennai, India the authors report an overall prevalence of 26.1% neuropathy in diabetic patients, with 24.1% of patients having associated retinopathy and 24.8% having nephropathy [18]. Lobo AC et al., reported that the prevalence of retinopathy was 12% and nephropathy was 40%, but this was assessed in patients with a duration of diabetes less than one year [19]. This was markedly different from study done by Bril V et al., where the prevalance of retinopathy was reported as 26% and nephropathy was found to be present only in 2% of patients with neuropathy.
In the present study the severity of retinopathy and nephropathy had a statistically significant correlation with the severity of diabetic neuropathy as assessed using TCNS score (p<0.001). These findings correspond to the results of study done by Weisman A et al., who also reported that the severity of diabetic retinopathy correlated with the severity of diabetic neuropathy [20]. Another study done by Liu F et al., on TCNS in DPN also concluded that severity of diabetic retinopathy and nephropathy went hand in hand with diabetic neuropathy [21].
Limitation
The present study had certain limitations, one being that it was a hospital based study and hence the results may not be generalized to the population on a community basis. The differences between patients with type 1 and type 2 diabetes were not compared.
Conclusion
From the present study, it can be concluded that, TCNS can be effectively used as a simple bedside screening tool to diagnose the presence of diabetic neuropathy and assess its severity in the Indian population. The duration of diabetes is more likely to have an effect on the severity of neuropathy than glycaemic control. Further, using the severity score of TCNS, the clinician can be alerted to the possibility of other co-existing microvascular complications such as retinopathy and nephropathy.
DN: Diabetic Neuropathy; NPDR: Non Proliferative Diabetic Retinopathy; PDR: Proliferative Diabetic Retinopathy.
[1]. Guariguata L, Whiting D, Hambleton I, Beagley J, Linnenkamp U, Shaw JE, Global estimates of diabetes prevalence for 2013 and projections for 2035Diabetes Research and Clinical Practice 2014 103(2):137-49. [Google Scholar]
[2]. Singh G, Chawla S, Amputation in diabetic patientsMedical Journal, Armed Forces India 2006 62(1):36-39. [Google Scholar]
[3]. Ashok S, Ramu M, Deepa R, Mohan V, Prevalence of neuropathy in type 2 diabetic patients attending a diabetes centre in South IndiaJ Assoc Physicians India 2002 50(4):546-50. [Google Scholar]
[4]. Gill HK, Yadav SB, Ramesh V, Bhatia E, A prospective study of prevalence and association of peripheral neuropathy in Indian patients with newly diagnosed type 2 diabetes mellitusJ Postgrad Med 2014 60(3):270-75. [Google Scholar]
[5]. Boulton A, Vinik A, Arezzo J, Bril V, Feldman EL, Freeman R, Diabetic Neuropathies: a statement by the American Diabetes AssociationDiabetes Care 2005 28(4):956-62. [Google Scholar]
[6]. Bansal V, Kalita J, Misra UK, Diabetic neuropathyPostgraduate Medical Journal 2006 82(964):95-100. [Google Scholar]
[7]. Al-Geffari M, Comparison of different screening tests for diagnosis of diabetic peripheral neuropathy in primary health care settingInternational Journal of Health Sciences 2012 6(2):127-34. [Google Scholar]
[8]. Yang Z, Chen R, Zhang Y, Huang Y, Hong T, Sun F, Scoring systems to screen for diabetic peripheral neuropathyCochrane Database of Systematic Reviews 2014 (Issue 3) [Google Scholar]
[9]. Mythili A, Kumar KD, Subrahmanyam KA, Venkateswarlu K, Butchi RG, A comparative study of examination scores and quantitative sensory testing in diagnosis of diabetic polyneuropathyInternational Journal of Diabetes in Developing Countries 2010 30(1):43-48. [Google Scholar]
[10]. Jayaprakash P, Bhansali A, Bhansali S, Dutta P, Anantharaman R, Shanmugasundar G, Validation of bedside methods in evaluation of diabetic peripheral neuropathyThe Indian Journal of Medical Research 2011 133(6):645-49. [Google Scholar]
[11]. Dixit S, Maiya A, Diabetic peripheral neuropathy and its evaluation in a clinical scenario: a reviewJ Postgrad Med 2014 60(1):33-40. [Google Scholar]
[12]. Bril V, Perkins B, Validation of the Toronto clinical scoring system for diabetic polyneuropathyDiabetes Care 2002 25(11):2048-52. [Google Scholar]
[13]. Bril V, Tomioka S, Buchanan R, Perkins B, Reliability and validity of the modified Toronto Clinical Neuropathy Score in diabetic sensorimotor polyneuropathyDiabetic Medicine 2009 26(3):240-46. [Google Scholar]
[14]. Bril V, Hirose T, Tomioka S, Buchanan R, Ranirestat for the Management of Diabetic Sensorimotor PolyneuropathyDiabetes Care 2009 32(7):1256-60. [Google Scholar]
[15]. Bostani A, Homayuonfar H, The Relationship between NCS Findings and Toronto clinical scoring system of neuropathy in diabetic polyneuropathyJournal of Kermanshah University of Medical Sciences 2006 [Google Scholar]
[16]. Sabanayagam C, Liew G, Tai ES, Shankar A, Lim SC, Subramaniam T, Relationship between glycated haemoglobin and microvascular complications: is there a natural cut-off point for the diagnosis of diabetes?Diabetologia 2009 52(7):1279-89. [Google Scholar]
[17]. Bansal D, Gudala K, Muthyala H, Esam HP, Nayakallu R, Bhansali A, Prevalence and risk factors of development of peripheral diabetic neuropathy in type 2 diabetes mellitus in a tertiary care settingJ Diabetes Invest 2014 5(6):714-21. [Google Scholar]
[18]. Pradeepa R, Rema M, Vignesh J, Deepa M, Deepa R, Mohan V, Prevalence and risk factors for diabetic neuropathy in an urban south Indian population: the Chennai Urban Rural Epidemiology Study (CURES-55)Diabetic Medicine 2008 25(4):407-12. [Google Scholar]
[19]. Lobo AC, George P, Fernandes KM, An assessment of the patterns and severity of diabetic neuropathy using the modified - Toronto Clinical Neuropathy Score in recently detected diabeticsInternational Journal of Biomedical Research 2017 8(05):266-70. [Google Scholar]
[20]. Weisman A, Bril V, Ngo M, Lovblom L, Halpern E, Orszag A, Identification and prediction of diabetic sensorimotor polyneuropathy using individual and simple combinations of nerve conduction study parametersPlos One 2013 8(3):e58783 [Google Scholar]
[21]. Liu F, Mao JP, Yan X, Toronto clinical scoring system in diabetic peripheral neuropathyZhong Nan Da Xue Xue Bao Yi Xue Ban 2008 33(12):1137-41. [Google Scholar]