Concomitant Pulmonary Embolism and Acute Renal Failure in a Patient Undergoing Head Neck Surgery: A Case Report and Review of Literature
Xin Yang1, Shalva R Gvetadze2, LV Mingming3, Jinbing Wang4, Jun Li5
1 Department of Oral Maxillofacial Head Neck Oncology, Ninth People’s Hospital, Jiao Tong University School of Medicine, Shanghai, China; Shanghai Key Laboratory of Stomatology and Shanghai Research Institute of Stomatology, National Clinical Research Center of Stomatology, Shanghai, China.
2 Department of Oral Maxillofacial Head Neck Oncology, Ninth People’s Hospital, Jiao Tong University School of Medicine, Shanghai, China; Shanghai Key Laboratory of Stomatology and Shanghai Research Institute of Stomatology, National Clinical Research Center of Stomatology, Shanghai, China; Central Research Institute of Dentistry and Maxillofacial Surgery, Moscow, Russia.
3 Department of Oral Maxillofacial Head Neck Oncology, Ninth People’s Hospital, Jiao Tong University School of Medicine, Shanghai, China; Shanghai Key Laboratory of Stomatology and Shanghai Research Institute of Stomatology, National Clinical Research Center of Stomatology, Shanghai, China.
4 Department of Oral Maxillofacial Head Neck Oncology, Ninth People’s Hospital, Jiao Tong University School of Medicine, Shanghai, China; Shanghai Key Laboratory of Stomatology and Shanghai Research Institute of Stomatology, National Clinical Research Center of Stomatology, Shanghai, China.
5 Department of Oral Maxillofacial Head Neck Oncology, Ninth People’s Hospital, Jiao Tong University School of Medicine, Shanghai, China; Shanghai Key Laboratory of Stomatology and Shanghai Research Institute of Stomatology, National Clinical Research Center of Stomatology, Shanghai, China.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Jun Li, Rd. Zhi Zhao Jv 639, 200011, Shanghai, China.
E-mail: 116004@sh9hospital.org, 13636616199@163.com
Surgical interventions for malignant neoplasms of the head and neck region bear a risk of general medical complications. Venous thromboembolism and pulmonary embolism is closely tied to patients suffering from carcinoma who are exposed to thrombotic events. These comprise the most common medical complications in patients undergoing head neck surgery. In patients with pulmonary embolism, the incidence of acute renal failure ranges is not rare. Rhabdomyolysis is an unusual and probably often overlooked clinical syndrome which may lead to acute renal failure and permanent myoglobinuric renal damage. Clinical case which is outlined here represents an example of pulmonary embolism combined with acute renal failure in a patient with no obvious risk factors after bilateral neck dissection. Rhabdomyolysis in such conditions may be an overlooked and underdiagnosed reason of acute renal failure development.
Head and neck cancer, Rhabdomyolysis, Venous thromboembolism
Case Report
A female patient, 67-year-old, was referred to the department with bilateral regional metastatic disease which progressed in four months after local excision of the primary hard palate lymphoepithelial carcinoma in another hospital. The patient had no significant history of smoking and alcohol intake, no abnormal respiratory or cardiac findings. Preoperative blood counts including D-dimer were all within normal limits. The physical condition was mildly asthenic with 161 cm height and 43 kg weight.
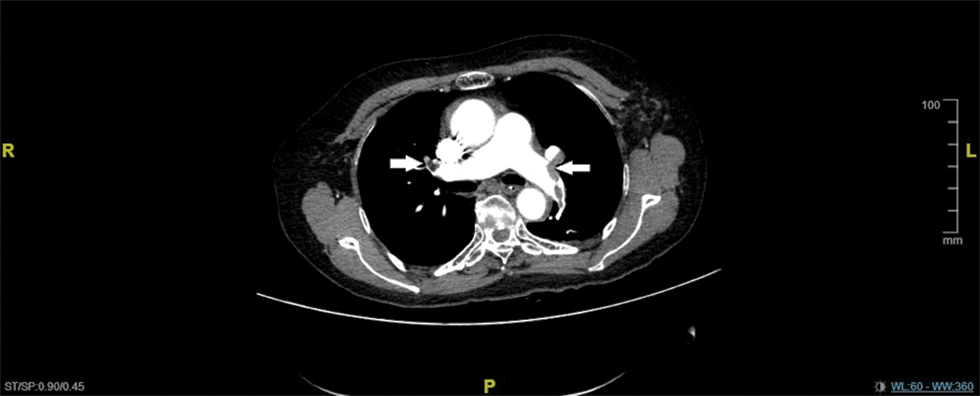
Bilateral radical modified functional neck dissection was undertaken, surgery duration – 2 h 25 min, anaesthesia time – 3 h 05 min, intraoperative blood loss – 350 ml. The first postoperative hours were uneventful with stable vital signs when a sudden drop of oxygen saturation and progression of dizziness, cold sweats and pale skin colour were observed. Twenty hours postoperatively, the patient was transferred to the Intensive Care Unit (ICU) for ventilation. On the second day postoperatively, hypoxia remained, myocardial infarction was initially suspected with CK and CK-MB serum levels markedly elevated. Electrocardiography (ECG) from lead I showed a prominent S wave with obvious Q and T waves from lead III, a fairly specific sign of PE. The patient was subjected to chest Computed Tomography Angiography (CTA) which revealed massive bilateral pulmonary vein thrombosis [Table/Fig-1]. Both pulmonary arteries were injected with 250,000 units of urokinase for establishing thrombolysis. The lumen of the pulmonary veins was seen and pulmonary arteries were visualized bilaterally.
CTA reveals massive bilateral pulmonary vein thrombosis (arrows).
Intravenous heparin was administered for three weeks with the dose adjusted to blood counts performance. After pulmonary artery thrombolysis, the patient was returned to the ICU where prominent dark-coloured haematuria was noticed. The patient was referred for Continuous Veno-Venous Haemodialysis (CVVHD) and total of 2000 ml ultrafiltration was achieved in the initial treatment day. In the presence of haematuria with intermittent anuria in the next few days, Acute Renal Failure (ARF) was diagnosed.
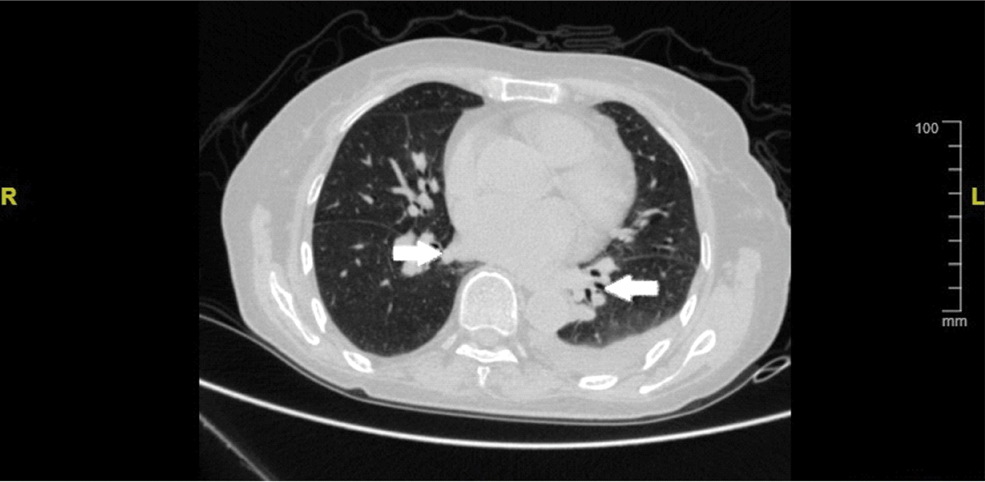
Kidney ultrasound and CT exams failed to unmask any obstruction of the genitourinary tract. Blood chemistry exhibited continuous hypokalaemia which was treated with cautious infusion therapy. Being under CVVHD for more than two weeks, the patient gradually developed normal urine output quality and quantity with an average of 2500 ml per day. Ventilator-assisted breathing was stopped three weeks postoperatively as repeating pulmonary CTA showed recovered lung and pleura condition with no emboli in the pulmonary veins [Table/Fig-2]. Overall she stayed in the hospital for more than 50 days with the majority of this time spent in the ICU. After discharge, the case was overviewed at a clinical group discussion and rhabdomyolysis was first suggested then as a probable cause of ARF.
Repeated pulmonary CTA showed recovered lung and pleura condition with no emboli bilaterally in the pulmonary veins (arrows).
Clinical follow up at three and six months after discharge revealed no signs of locoregional recurrence as well as no complains on the general health of the patient.
Discussion
Surgery is a treatment of choice in the majority of head and neck malignancies. Depending on the primary and regional stage of the disease the extent of the surgical intervention may increase significantly which in turn increases the risk of postoperative complications. The rate of general postoperative complications in patients with head and neck tumours undergoing radical resections was reported to be up to 26%, Pulmonary Embolism (PE) being the most common one [1]. Preoperative comorbidities were shown to be related to the rate of medical and surgical complications [2]. Generally it is estimated that surgery-related complications can be expected with advanced staged tumours when prolonged, complex surgery is indicated. Medical, non-surgical complications are linked to elderly patients with a history of cardiovascular or endocrine disorders [3]. Respiratory complications as pneumonia or atelectasis and acute cardiologic events are all not seldom in the postoperative period in head neck surgery patients especially, in those requiring free flap reconstruction [4,5]. In subjects with no relevant pre-treatment health conditions, gastrointestinal or genitourinary postoperative complications are rarely observed.
Venous Thromboembolism (VTE) is closely tied to patients suffering from carcinoma who are exposed to thrombotic events with much higher danger related to a number of established factors. These include advanced age, necessity for free flap reconstruction, longer immobilization, congestive heart failure, history of smoking and alcohol abuse and chemotherapy [6]. The latest reported rate of VTE in patients undergoing oral and maxillofacial oncologic surgery is 0.14% [7]. The incidence of VTE and PE in patients subjected to head and neck operations for malignant neoplasms is 6 times higher to that of those undergoing general otolaryngologic procedures (0.6% vs. 0.1%) [8]. For diagnosing PE, pulmonary CTA is currently the most frequently used imaging technique [9]. This modality is deemed as a fast and reliable mean for excluding or confirming PE. Attentive risks and comorbidities consideration is necessary for refining the clinical value while minimizing the potential harm from overutilization and overdiagnosis [10]. Additionally, certain ECG changes may be helpful in confirming massive PE events [11]. Several strategies of prevention and treatment of PE with various anticoagulation agents in different clinical settings were evaluated and successfully utilized [12,13].
ARF is an abrupt decline in renal function sufficient to cause retention of nitrogenous waste. The severity of the trauma, the magnitude of the surgical procedure, the gravity of the underlying medical conditions that are linked with ARF all predispose to possible multiorgan failure, which causes high mortality rate. ARF still is associated with high mortality rates, ranging from 25% to 90% [14,15]. Whether or not oliguria is present, a progressive rise in serum creatinine in the posttrauma or postoperative period should suggest the presence of ARF. Because ARF represents one of the few incidences of completely reversible severe organ failure, early intensive and continuing treatment is indicated. In patients with PE the incidence of ARF is not rare with rates up to to 13% reported in the literature [16]. Severe renal impairment may be a result of cardio-renal syndrome which in turn is a known sequence of PE. Lately it was reported that massive PE, sepsis, anaemia, diabetes mellitus, hypertension, and chronic kidney disease are strong predictors of ARF [17]. Management of ARF is primary in-time initiation of continuous renal replacement therapy which can be implied in different modalities and bears high cure rates, re-establishing renal function.
Rhabdomyolysis is an unusual and probably often overlooked clinical syndrome which may lead to ARF and permanent myoglobinuric renal damage. This condition may be observed after muscle ischemia which is caused by compression of the lumbar and pelvic muscles resulted in muscle injury. Serum CK levels (normally less than 85 U/L) are markedly elevated electrolyte disturbances may include hypocalcaemia and hyperkalaemia, hypovolaemia, metabolic acidosis may as well take place [18]. Factors associated with the disease can be categorized into five groups. Those include: disorders of energy production (convulsive seizures); metabolic depression (diabetes mellitus, prolonged coma); infection; toxins and muscle injury. Subclinical rise of creatine kinase may be the expression of rhabdomyolysis that can present as a medical emergency such as ARF, compartment syndrome, cardiac dysrhythmias and disseminated intravascular coagulopathy. The main pathophysiological mechanisms of myoglobinuric-related ARF are renal vasoconstriction, formation of intraluminal casts and direct cytotoxicity promoted by heme-protein [19]. A timely recognition is crucial for treatment of rhabdomyolysis. In the acute stage of the disease, management should be focused on retaining renal function, improving metabolism, and volume replacement. Otherwise, ARF is likely to develop which necessitates haemodialysis treatment initiation. Usually the affected patients exhibit only one event of rhabdomyolysis.
The presented clinical case describes a concomitant massive PE and ARF which were sudden early events that progressed in the first and second postoperative days, respectively. There are four possible explanations to relate PE and ARF. First, in patients with massive PE, pulmonary hypertension and heart failure will occur. An unstable haemodynamic status, hypoperfusion and hypoxia can lead to renal dysfunction. This pathway may be deemed as reasonable explanation of ARF development in our clinical case. Second issues are ARF risk factors which predispose to acute renal complications during or after surgery. These are diabetes mellitus, hypertension and chronic kidney illness. Thirdly, prerenal azotemia may be induced by third-space fluid loss after PE and finally, anaemia respiratory failure and sepsis may contribute to ARF [17]. During preoperative assessment our patient failed to demonstrate any significant comorbidity and was haemodynamic stable throughout the whole period of hospitalization. The likeliness of rhabdomyolysis being the cause of ARF is proposed by elevated CK and CK-MB test levels (712 ng/mL) which were performed in suspicious of a heart failure as CK-MB is a factor sensitive to myocardial infarction which was not confirmed. Rhabdomyolysis may have been triggered by an intraoperative cushion pressure to the pelvic region as in one of the patient described by Dequanter D et al., [18].
Conclusion
The clinical case which is outlined here represents an example of postoperative PE combined with ARF in a patient with no obvious risk factors after bilateral neck dissection. Rhabdomyolysis in such conditions may be an overlooked and underdiagnosed reason of ARF development.
Authors Contribution
Xin Yang (XY), Shalva R Gvetadze (SRG), Mingming Lv (ML), Jinbing Wang (JW), Jun Li (JL).
XY and SRG collected the clinical data, analysed the clinical test results, diagnosed the disease and prepared the manuscript. ML took part in the management of the patient, JW took part in the operation and arranged the postoperative care, all this work was conducted under supervision of JL. Considering the work load, the authors XY and SRG contributed equally to this work.
[1]. McMahon JD, Maclver C, Smith M, Stathopoulos P, Wales C, McNulty R, Postoperative complications after major head and neck surgery with free flap repair – prevalence, patterns, and determinants: a prospective cohort studyBr J Oral Maxillofac Surg 2013 51(8):689-95. [Google Scholar]
[2]. Ferrier MB, Spuesens EB, Le Cessie S, de Jong RJB, Comorbidity as a major risk factor for mortality and complications in head and neck surgeryArch Otolaryngol Head Neck Surg 2005 131(1):27-32. [Google Scholar]
[3]. Peters TA, Boukje AC, van Dijk, van der Laan BF, Halmos GB, Relation between age, comorbidity, and complications in patients undergoing major surgery for head and neck cancerAnn Surg Oncol 2014 21(3):963-70. [Google Scholar]
[4]. Lin HW, Bhattacharyya N, Contemporary assessment of medical morbidity and mortality in head and neck surgeryOtolaryngol Head Neck Surg 2011 146(3):385-89. [Google Scholar]
[5]. Pohlenz P, Klatt J, Schmelzle R, Li L, The importance of in-hospital mortality for patients requiring free tissue transfer for head and neck oncologyBr J Oral Maxillofac Surg 2013 51(6):508-13. [Google Scholar]
[6]. Pannucci CJ, Barta RJ, Portschy PR, Dreszer G, Hoxworth RE, Kalliainen LK, Assessment of postoperative venous thromboembolism risk in plastic surgery patients using 2005 and 2010 Carpini risk scorePlast Reconstr Surg 2012 130(2):343-53. [Google Scholar]
[7]. Wang Y, Liu J, Yin X, Hu J, Kalfarentzos Zhang C, Venus thromboembolism after oral and maxillofacial oncologic surgery: Report and analysis of 14 cases in Chinese populationMed Oral Patol Oral Cir Bucal 2017 22(1):e115-21. [Google Scholar]
[8]. Moreano EH, Hutchison JL, McCulloch TM, Graham SM, Funk GF, Hoffman HT, Incidence of deep venous thrombosis and pulmonary embolism in otolaryngology-head and neck surgeryOtolaryngol Head Neck Surg 1998 118(6):777-84. [Google Scholar]
[9]. Kincl V, Feitova V, Panovsky R, Stepanova R, Assessment of severity of acute pulmonary embolism using CT pulmonary angiography parametersBiomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2015 159(2):259-65. [Google Scholar]
[10]. Albrecht MH, Bickford MW, Nance JW Jr, Zhang L, De Cecco CN, Wichmann JL, State-of-the-art pulmonary CT angiography for acute pulmonary embolismAJR Am J Roentgenol 2017 208(3):495-504. [Google Scholar]
[11]. Kanzaki S, Kunihiro T, Imanishi T, Yamashita D, Ogawa K, Two cases of pulmonary embolism after head and neck surgeryAuris Nasus Larynx 2004 31(3):313-17. [Google Scholar]
[12]. Francis CW, Prevention of venous thromboembolism in hospitalized patients with cancerJ Clin Oncol 2009 27(29):4874-80. [Google Scholar]
[13]. Song JQ, Xuan LZ, Wu W, Huang JF, Zhong M, Low molecular weight heparin once versus twice for thromboprophylaxis following esophagectomy: a randomized, double-blind and placebo-controlled trialJ Thorac Dis 2015 7(7):1158-64. [Google Scholar]
[14]. Clermont G, Acker CG, Angus DC, Sirio CA, Pinsky MR, Johnson JP, Renal failure in the ICU: comparison of the impact of acute renal failure and end-stage renal disease on ICU outcomeKidney Int 2002 62(3):986-96. [Google Scholar]
[15]. Siegel NJ, Shah SV, Acute renal failure: directions for the next decadeJ Am Soc Nephrol 2003 14(8):2176-77. [Google Scholar]
[16]. Goldhaber SZ, Visani L, De Rosa M, Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER)Lancet 1999 353(9162):1386-89. [Google Scholar]
[17]. Chang CH, Fu CM, Fan PC, Chen SW, Chang SW, Mao CT, Acute kidney injury in patients with pulmonary embolism: a population-based studyMedicine (Baltimore) 2017 96(9):e5822 [Google Scholar]
[18]. Dequanter D, Vercruysse N, Shahla M, Paulus P, Lothaire P, Rhabdomyolysis in head and neck surgeryB-ENT 2014 10(3):171-73. [Google Scholar]
[19]. Zutt R, van der Kooi AJ, Linthorst GE, Wanders RJ, de Visser M, Rhabdomyolysis: review of literatureNeuromuscul Disord 2014 24(8):651-58. [Google Scholar]