Solitary Intramammary Plexiform Schwannoma Mimicking Phyllodes Tumour on Cytology
Shveta1, Kiran Agarwal2, Ankita Sharma3
1 Senior Resident, Department of Pathology, Lady Hardinge Medical College, New Delhi, India.
2 Director Professor, Department of Pathology, Lady Hardinge Medical College, New Delhi, India.
3 Postgraduate Student, Department of Pathology, Lady Hardinge Medical College, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Shveta, 46-Brothers Apartment, IP Extension, Patparganj, New Delhi-110092, India.
E-mail: shvetawadhwa05@gmail.com
Plexiform schwannoma is a rare subtype of schwannoma with no case reported in the breast to the best of our knowledge. We report a case of intramammary plexiform schwannoma in a 20-year-old female which was diagnosed as Phyllodes tumour on cytology. Marginal resection is curative. This diagnosis show be kept in mind when reporting on Fine Needle Aspirates of stromal lesions of breast.
Breast, Cytologic diagnosis, Rare, S100
Case Report
A 20-year-old female presented to Surgery outpatient Department with complaint of pain and lump in the left breast for past 4.5 years. The female was unmarried, non-pregnant and non-lactating. There was no history or family history of neurofibromatosis in the patient. Bilateral breast ultrasound revealed multinodular heterogenous hypoechoic lesion in the left breast with well defined boundaries and posterior acoustic enhancement. The features were suggestive of a fibroadenoma.
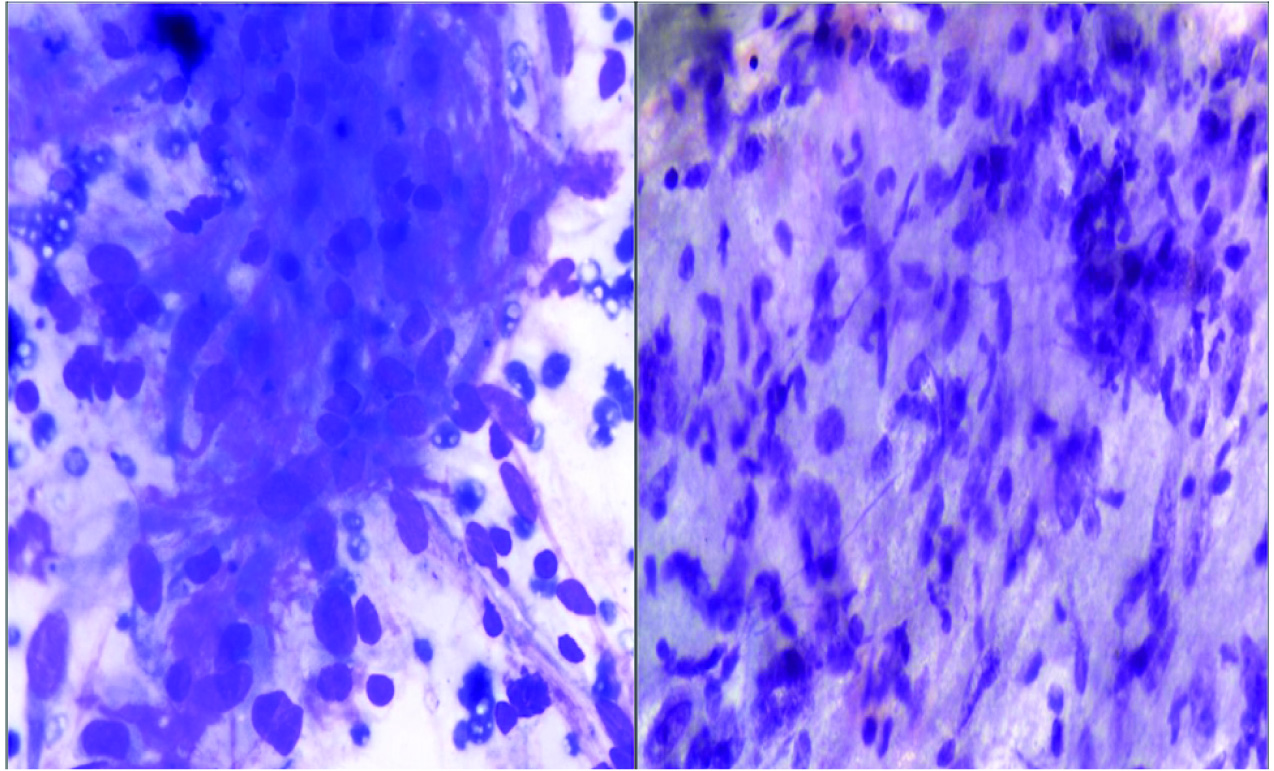
On examination, a slightly mobile lump measuring 6 cm x 5 cm x 4.5 cm was felt in the upper outer quadrant of left breast. The mass appeared fixed to underlying chest wall but not to the skin. No skin changes or nipple discharge was seen. No axillary lymphadenopathy was felt. Clinically, it looked like a case of benign breast disease. Fine Needle Aspiration (FNAC) was performed with a 22 Gauge needle which showed many cellular stromal fragments with oval to elongated cells having ill defined cytoplasmic borders [Table/Fig-1]. Occasionally, nuclear palisade was seen. The nuclei showed anisonucleosis with coarse chromatin in some. Few bizarre nuclei were seen. Bare nuclei were present in the background. No breast ductal epithelium was seen. Possibility of Phyllodes tumour was suggested because of the site, clinical and pathological features. However, urgent excision was advised owing to nuclear atypia.
Cytology smears showing oval to elongated cells with moderate anisonucleosis (Giemsa 40X).
Our patient underwent a preoperative tru-cut biopsy before an excisional biopsy. Tru-cut biopsy showed spindle cells arranged in short fascicles with few myxoid areas and focal anisonucleosis. No other architectural pattern was seen. On tru-cut biopsy, a possibility of stromal lesion or tumour was suggested. Immunohistochemistry (IHC) showed Vimentin positivity, Estrogen Receptor (ER) negativity, Progesterone Receptor (PR) negativity, Epithelial Membrane Antigen (EMA) negativity and Ki67 <1%. A diagnosis of stromal tumour was made.
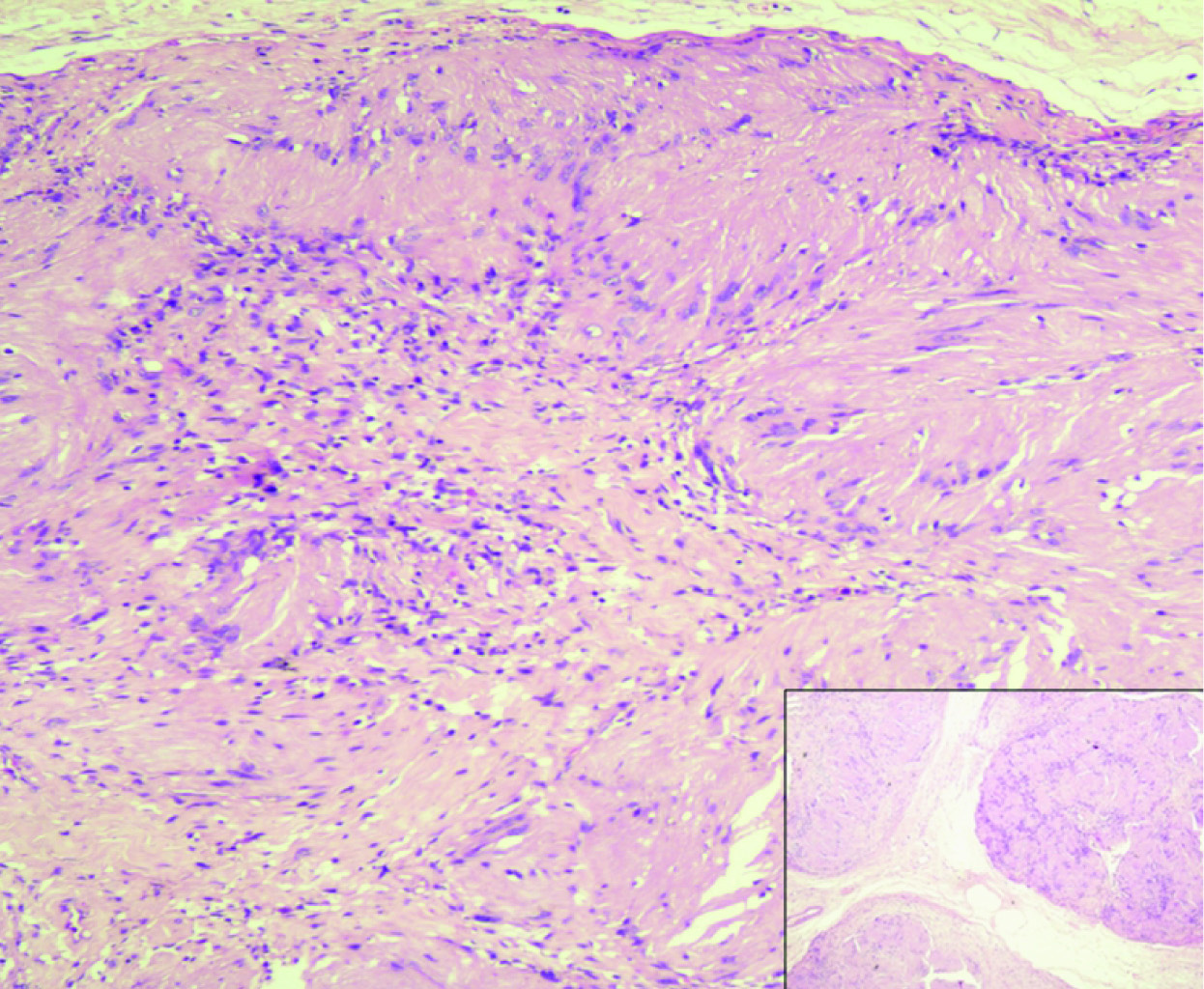
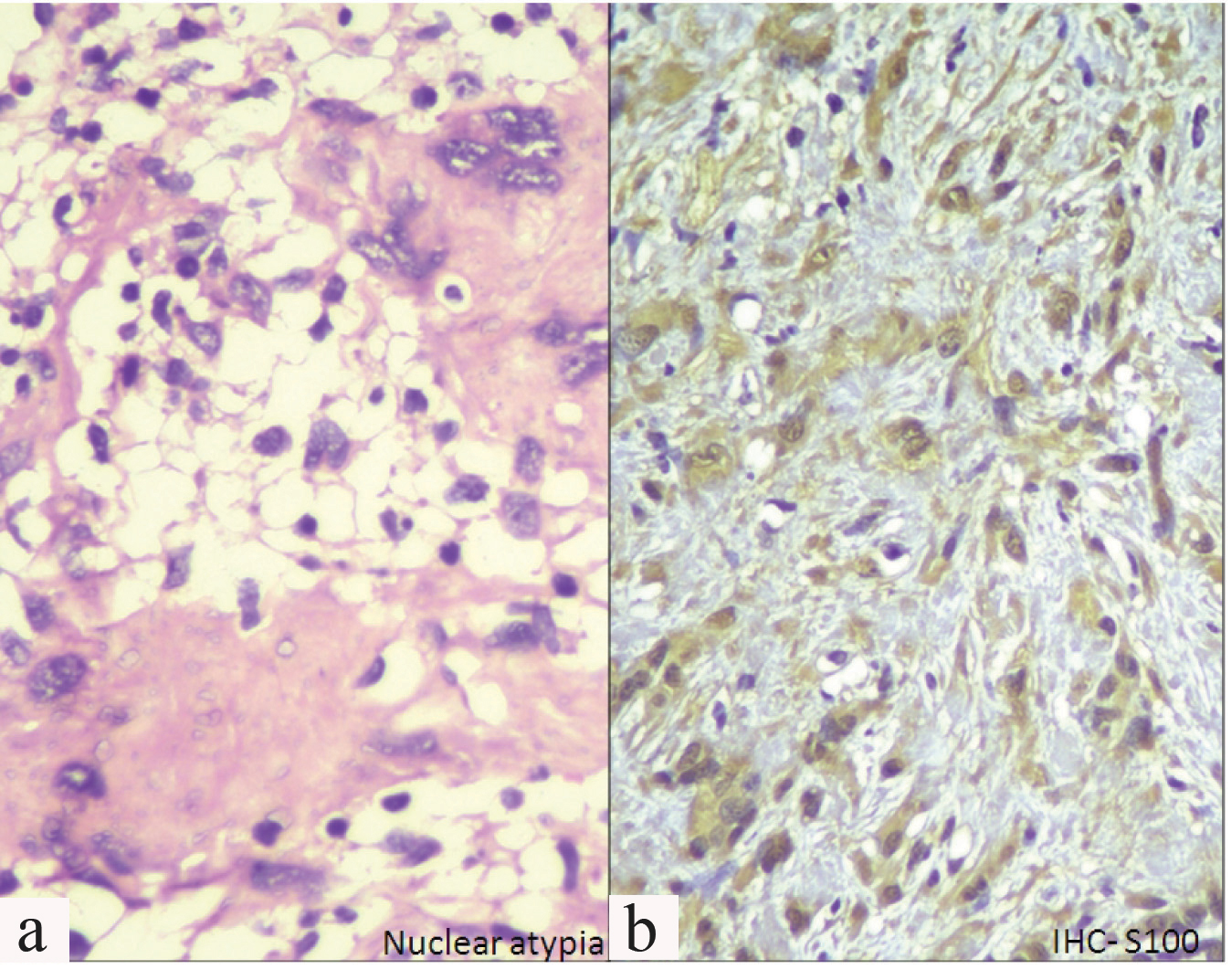
Lumpectomy was performed and a formalin fixed specimen comprising of multiple grey white bits ranging from 1 cm to 2.8 cm in greatest dimension was received. Histopathology showed well defined round masses of spindle cells separated by fibrous septa. These spindle shaped cells showed nuclear atypia with mitosis 1-2/hpf (high power field). Spindle cells were mostly arranged in a palisading fashion and an organoid arrangement (Verocay bodies). Hypercellular Antoni A and Hypocellular Antoni B areas were seen [Table/Fig-2]. Focal areas showing myxoid change and few dilated congested blood vessels were seen. Few cells showed bizarre nuclei [Table/Fig-3a]. No areas of necrosis were identified. Surrounding breast tissue was clearly demarcated and showed normal morphology. Morphology along with S100 positivity [Table/Fig-3b] confirmed the final diagnosis of plexiform schwannoma. Vimentin positivity, ER negativity, PR negativity, EMA negativity and Ki 67 <1% had already been demonstrated on the tru-cut biopsy.
Antoni A and Antoni B areas with verocay bodies, inset - nodular/plexiform pattern (H&E 10X, INSET – H&E – 4X).
a) Bizzare nuclei (H&E 40X); b) IHC - S100 positivity (40X).
Hence, a final diagnosis of plexiform schwannoma was given. Patient has been followed up till 24 months after excision and is asymptomatic.
Discussion
Schwannoma is a benign tumour of peripheral nerves arising from the schwann cells. Schwannoma in the breast is a very rare tumour with 31 cases reported till date [1]. Das Gupta TK et al., reported the percentage of schwannomas arising in the breast to be only 2.6% of all schwannomas [2]. Plexiform schwannoma is a rare subtype of schwannoma (5% of all schwannomas) showing a multinodular pattern of growth [3]. Hence, the incidence of plexiform schwannomas in breast is extremely rare. There have been cases where, chest wall schwannomas have been mistaken for malignant soft tissue tumours of the breast because of the rarity of the lesion and failure to consider it as a differential in case of breast lumps [4]. Rare deep seated plexiform schwannomas have been reported in extremities, retroperitoneum, pelvis, parotid, vulva and esophagus. We also, in our case did not consider the possibility of schwannoma in a breast on FNAC and tru-cut biopsy. The diagnosis was made on excision specimen, when a larger sample was available for microscopic examination and architectural features were well visible.
Yaman I et al., mention only 30 cases of breast schwannomas till date [1]. A mean age of 48.6 years and a median age of 45 years have been reported [5]. In the present case, the patient was comparatively young with diagnosis and excision of schwannoma at only 20 years of age. A similar case was reported by Bernardello F et al., where the age of the patient was 18 years [6].
Schwannomas are known to occur post radiation therapy in 10% of cases [7]. Our patient did not have any significant history of exposure to radiation.
Schwannomas are reported to be well demarcated from the main breast tissue and well encapsulated which is also seen in the present study. Ultrasonographically, do not show any specific features. Hence, difference from fibroadenoma, phyllodes, breast cancers and even other mesenchymal tumours is difficult to make [5].
Cytologically breast schwannomas are difficult to differentiate from phyllodes tumour [8], myoepithelioma and other mesenchymal tumours of the breast. Findings in cytology smears also depend upon the area being examined i.e., Antoni A or Antoni B areas. Phyllodes tumour can show sheets of ductal epithelium in addition to spindle cells. Other fibromatous and smooth muscle tumours also show spindle cells in a palisading fashion. Verocay bodies are the only specific features for a schwannoma [9]. S100 staining also helps in differentiating between them, but myoepithelioma also stains positive for S100 protein on IHC [10]. Verocay bodies and S100 help in the final diagnosis of schwannoma in those cases.
In our case, there was presence of atypical cells, nuclear anisonucleosis and coarse chromatin in few cells. Hence, exclusion of malignancy was not possible on cytology. Plexiform schwannomas are known to have increased cellularity, mild to moderate pleomorphism, mitotic activity till 1-10/10 HPF with focal necrosis and myxoid change [11].
Recurrence of schwannoma after surgical excision has not been reported and the malignant transformation is extremely rare, although a local recurrence can follow an incomplete resection [5].
It is very important to differentiate them from plexiform neurofibromas, as plexiform neurofibromas are virtually pathognomonic of neurofibromatosis Type 1 and have a propensity for malignant transformation [12]. Such transformation to malignant peripheral nerve sheath tumour is rare but is associated with a poor prognosis, with a five-year survival rate of 34% [13].
Conclusion
To the best of our knowledge, this is the first case reported of a plexiform schwannoma in breast. We highlight the occurrence of a rare plexiform subtype of schwannoma in the breast which is the rarest of rare location. Pathologists should make sure to take its occurrence into account especially, when evaluating cytological smears. Although, histopathological examination gives a confirmatory diagnosis, marginal resection can be performed after cytological diagnosis and appears to be adequate for treatment.
[1]. Yaman I, Derici H, Schwannoma of the breast: A rare caseThe Journal of Breast Health 2013 9(3):172-74. [Google Scholar]
[2]. Das Gupta TK, Brasfield RD, Strong EW, Hajdu SI, Benign solitary schwannomas (neurilemomas)Cancer 1969 24(2):355-66. [Google Scholar]
[3]. Val-Bernal J, Figols J, Vázquez-Barquero A, Cutaneous plexiform schwannoma associated with neurofibromatosis type 2Cancer 1995 76(7):1181-86. [Google Scholar]
[4]. Datta S, A rare case of chest wall schwannoma with destruction of rib, masquerading as a breast massJ Clin Diagn Res 2014 8(6):FD01-02. [Google Scholar]
[5]. Raina B, Misri A, Singh G, Chrangoo RK, A rare peripheral nerve tumour’ schwannoma’ in the breastJK Science 2014 16(1):37-39. [Google Scholar]
[6]. Bernardello F, Caneva A, Bresaola E, Mombello A, Zamboni G, Bonetti F, Breast solitary schwannoma: Fine-needle aspiration biopsy and immunocytochemical analysisDiagnostic cytopathology 1994 10(3):221-23. [Google Scholar]
[7]. Dialani V, Hines N, Wang Y, Slanetz P, Breast schwannoma: a case reportCase Reports in Medicine 2011 :930841 [Google Scholar]
[8]. Thejaswini MU, Padmaja KP, Srinivasamurthy V, Rao MS, Solitary intramammary schwannoma mimicking phylloides tumour: Cytological clues in the diagnosisJournal of Cytology/Indian Academy of Cytologists 2012 29(4):258 [Google Scholar]
[9]. Shah AA, Latoo S, Ahmad I, Malik AH, Singh AP, Hassan S, Schwannoma causing resorption of zygomatic archJ Oral Maxillofac Pathol 2011 15(1):80-84. [Google Scholar]
[10]. Wilson AG, Hofmeister EP, Thompson M, Recurrent schwannoma with bony erosion of the distal middle fingerAm J Orthop 2007 36(3):37-39. [Google Scholar]
[11]. Goldblum JR, Weiss SW, Folpe AL, Enzinger and Weiss’s Soft Tissue Tumours E-BookElsevier Health Sciences 2013 Oct 11 [Google Scholar]
[12]. Val-Bernal JF, Figols J, Vázquez-Barquero A, Cutaneous plexiform schwannoma associated with neurofibromatosis type 2Cancer 1995 76(7):1181-86. [Google Scholar]
[13]. Nguyen C, Kettler MD, Swirsky ME, Miller VI, Scott C, Krause R, Male breast disease: pictorial review with radiologic-pathologic correlationRadiographics 2013 33(3):763-79. [Google Scholar]