Mammary Analogue Secretory Carcinoma of Salivary Gland – A Diagnostic Dilemma
Chetan S Chaudhari1, Shruti Chandrakar2, Ganesh R Kshirsagar3, Prashant V Kumavat4, Nitin M Gadgil5
1 Assistant Professor, Department of Pathology, Lokmanya Tilak Municipal Medical College, Mumbai, Maharashtra, India.
2 Senior Resident, Department of Pathology, Lokmanya Tilak Municipal Medical College, Mumbai, Maharashtra, India.
3 Assistant Professor, Department of Pathology, Lokmanya Tilak Municipal Medical College, Mumbai, Maharashtra, India.
4 Assistant Professor, Department of Pathology, Lokmanya Tilak Municipal Medical College, Mumbai, Maharashtra, India.
5 Associate Professor, Department of Pathology, Lokmanya Tilak Municipal Medical College, Mumbai, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Shruti Chandrakar, 34, A/1, Maulshree Vihar, VIP Road, Raipur-492006, Chhattisgarh, India.
E-mail: shrutichandrakar9388@gmail.com
Mammary Analogue Secretory Carcinoma (MASC) of salivary gland is a recently described entity with unique morphologic, clinical and genetic characteristics, including the characteristic t(12;15)(p13;q25) with ETV6-NTRK3 translocation found in secretory carcinomas of the breast. Before their initial description, these salivary gland tumours were generally diagnosed as acinic cell carcinoma or adenocarcinoma. We present a case of 32-year-old, male presenting with painless swelling over left cheek which was gradually increasing in size. The histopathological examination of the lesion was done and two possible differential diagnosis were acinic cell carcinoma (papillary cystic variant) and MASC of the parotid gland. Immunohistochemistry was performed which demonstrated combined positivity for S-100, mammglobin, CK-7 and DOG-1 negativity which confirmed the diagnosis of MASC of parotid.
Acinic cell, Adenocarcinoma, Histopathological
Case Report
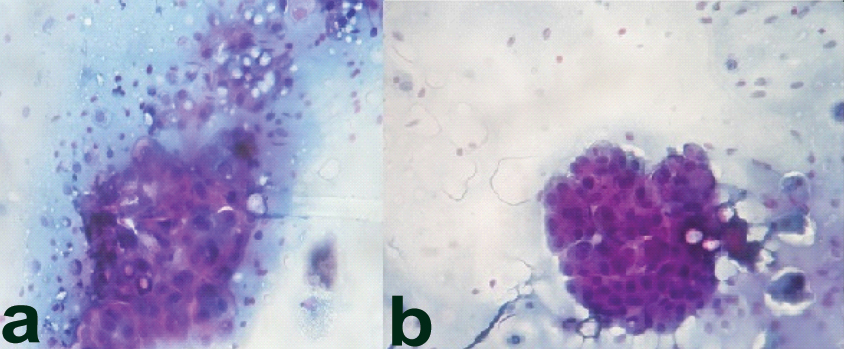
We present a case of 32-year-old male patients, who presented with a painless swelling over left cheek, since six months gradually increasing to present size of 4 cm × 4 cm × 3 cm. The swelling was non fluctuant and adhered to the underlying structures. Ultrasonography (USG) revealed left parotid showing well defined heterogeneously hypoechoic lesion in posterior lobe, measuring 3.5 cm × 3.5 cm suggestive of neoplastic aetiology. Fine Needle Aspiration Cytology (FNAC) of the lesion revealed sheets of bland polygonal epithelial cells [Table/Fig-1a,b] with eccentrically located small round uniform nucleus, small nucleoli and finely eosinophilic granular cytoplasm.
a) Sheets and clusters of bland polygonal epithelial cells (PAP 100X); b) Nuclei are uniform, small, round, eccentrically located, with small nucleoli. (PAP 100X).
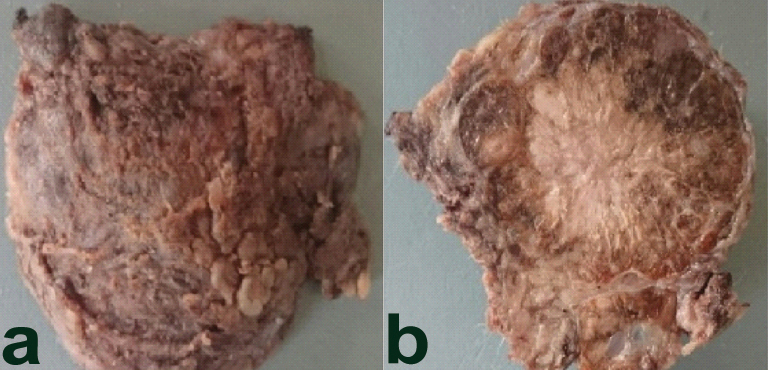
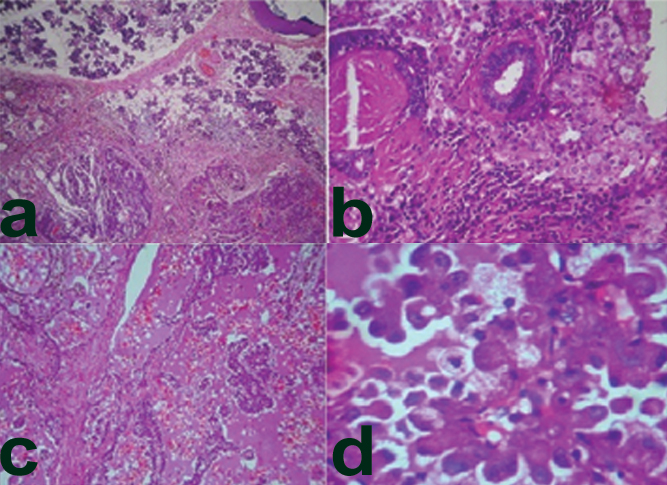
Surgical resection was done and the left parotidectomy specimen was received measuring 3.5 cm × 3.5 cm and was well encapsulated [Table/Fig-2a]. Cut surface revealed a well demarcated mass (3.0 cm × 3.0 cm) solid, greyish white with few peripherally placed cyst like areas surrounded by normal parotid gland, circumferentially [Table/Fig-2b]. Microscopy revealed a tumour with a mixture of micro papillary, microcytic [Table/Fig-3a,b] and solid patterns of growth with an overall lobulated architecture. The microcystic spaces contained eosinophilic bubbly secretions and the micropapillary growth pattern [Table/Fig-3c] consisting of tumour cells surrounded by fibrovascular core in a typical ‘hobnail’ pattern. The tumour cells had mild nuclear pleomorphism with abundant eosinophilic vacuolated cytoplasm [Table/Fig-3d] no necrosis, perineural, or lymphovascular invasion was seen. Based on the above findings two differentials were considered: acinic cell carcinoma (papillary cystic variant) and MASC of the parotid.
a) External surface of the tumour well encapsulated; b) Cut Surface: Well demarcated mass (3.0 cm × 3.0 cm) Solid, greyish white with few peripheral microcystic areas.
a) Microcystic, papillary structures with a lobulated architectural pattern H&E 40X); b) Tubular pattern (H&E 100X); c) Abundant eosinophilic, bubbly colloid-like secretions (H&E 10X); d) Round to oval vesicular nuclei with centrally located nucleoli. Finely granular eosinophilic and multi-vacuolated cytoplasm (H&E 40X).
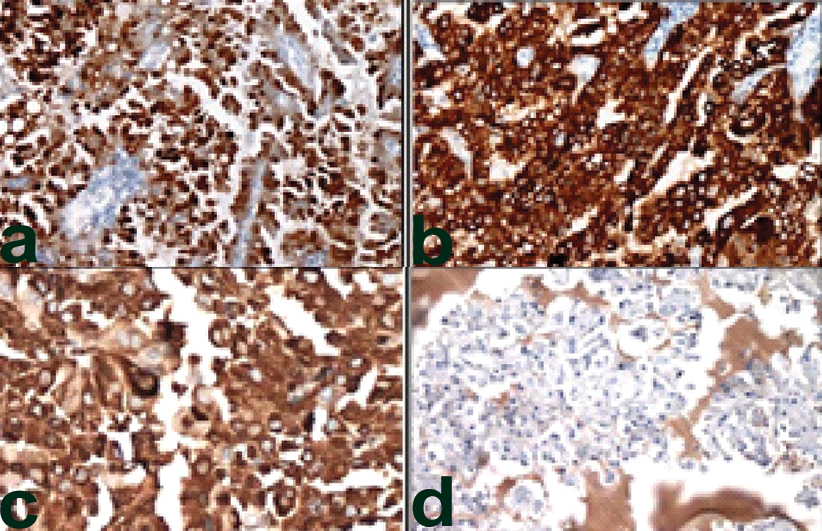
Immunohistochemistry was performed which showed combined positivity for S-100 [Table/Fig-4a] which was strong and diffusely positive, cytokeratin 7 [Table/Fig-4b] and mammaglobin [Table/Fig-4c], while DOG1/ANO1 were negative (which are positive in cases of acinic cell carcinoma) [Table/Fig-4d]. The final diagnosis of mammary analogue secretory carcinoma was made and unfortunately the patient was lost to follow up, so further treatment history could not be traced.
a) Strong and diffusely positive S-100 (40X); b) strong and diffusely positive CK-7 (40X); c) strong and diffusely positive Mammaglobulin (40X); d) DOG negative (40X).
Discussion
Salivary gland tumours are relatively rare malignancies with a worldwide annual incidence of 3 per 100,000 people [1]. Mammary MASC is a recently described salivary tumour that shares morphological features of secretory carcinoma of the breast, salivary acinic cell carcinoma, low grade cyst adenocarcinoma and mucoepidermoid carcinoma [2]. Before its discovery, MASC was often misinterpreted as acinic cell carcinoma due to the similarities in their morphologic appearance [3]. In 2010, Skálová A et al., distinguished MASC from acinic cell carcinoma based on distinctive molecular alteration. The ETV6-NTRK3 gene fusion, caused by a genetic translocation involving chromosomes 15 and 12, specifically t (12; 15) (p13; q25), has become a hallmark factor for differentiating MASC tumours from other salivary gland carcinomas [4].
Chiosea SI et al., has noted a male predominance [5]. The average age reported is 44.2 years (range, 14 to 77) [5,6] while our patient was a 32-year-old male. Chiosea SI et al., and Griffith C et al., observed that the majority (71%) of tumours were located in the parotid gland, similar to our case while the remainder occurred in a large diversity of locations, including the submandibular gland (7%), soft palate, buccal mucosa, base of tongue, and lips [5,6]. The average tumour size was 1.98 cm (range: 0.5 to 5.5 cm) as observed by Chiosea SI et al., and Griffith C et al., [5,6]. The tumour size in our case was larger than the average size. The most common presentation is slowly enlarging and painless nodule, often detected incidentally on physical examination, similar to our case [1,5-7]. There is a considerable overlap between acinic cell carcinoma and MASC, as both exhibit acinar differentiation and intercalated duct-type cells. The distinguishing feature is presence of blue-purple cytoplasmic zymogen granules, a diagnostic clue of serous acinar differentiation seen in acinic cell carcinoma [5-7] which was clearly absent in our case. In 2012, Chiosea SI et al., identified 17 “zymogen-poor” cases of acinic cell carcinoma from institutional archives. Of these, 10 were reclassified as MASC [5].
MASC stains positive for S100, contrary to the fact that acinic cell carcinoma is not positive for mammaglobin and mucin production is a feature in MASC, but not in acinic cell carcinoma [1,2,5-7]. similar to our case. Mucin production and differentiation into serous cells may lead to diagnostic confusion between MASC and mucoepidermoid carcinoma. However, MASC does not contain the histological features of combination of goblet-type mucous cells, intermediate and squamoid/epidermoid cells which are a characteristic of mucoepidermoid carcinoma [5,7]. While mucoepidermoid carcinoma is always positive for p63, S100 staining is an uncommon finding in these carcinomas [7].
Conclusion
Mammary Analogue Secretory Carcinoma is a newly described entity which should be kept in mind while dealing with salivary gland tumours that morphologically mimic other neoplasms like acinic cell carcinomas. They differ from conventional acinic cell tumours in immunohistochemical and molecular profile. Combined positivity for immunohistochemistry panel mammaglobin and S100, and negativity for DOG1/ANO1 are useful screening tools. Although, it appears to follow an indolent course in most patients, certain cases appear predisposed to distant metastasis and increased mortality. Currently, there is no way to predict which tumours will behave aggressively. Thus, a high index of suspicion should be kept while dealing with salivary gland tumours while keeping the differential of MASC in mind.
[1]. Parkin DM, Ferlay J, Curado MP, Bray F, Edwards B, Shin HR, Fifty years of cancer incidence: CI5 I-IXInt J Cancer 2010 127(12):2918-27. [Google Scholar]
[2]. Tognon C, Knezevich SR, Huntsman D, Roskelley CD, Melnyk N, Nathers JA, Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinomaCancer Cell 2002 2(5):367-76. [Google Scholar]
[3]. Chiosea SI, Griffith C, Assaad A, Seethala RR, The profile of acinic cell carcinoma after recognition of mammary analog secretory carcinomaAm J Surg Pathol 2012 36(3):343-50. [Google Scholar]
[4]. Skálová A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumour entityAm J Surg Pathol 2010 34(5):599-608. [Google Scholar]
[5]. Chiosea SI, Griffith C, Assaad A, Seethala RR, Clinicopathological characterization of mammary analogue secretory carcinoma of salivary glandsHistopathology 2012 61(3):387-94. [Google Scholar]
[6]. Griffith C, Seethala R, Chiosea SI, Mammary analogue secretory carcinoma: a new twist to the diagnostic dilemma of zymogen granule poor acinic cell carcinomaVirchows Arch 2011 459(1):117-18. [Google Scholar]
[7]. Griffith CC, Stelow EB, Saqi A, Khalbuss WE, Schneider F, Chiosea SI, The cytological features of mammary analogue secretory carcinoma: a series of 6 molecularly confirmed casesCancer Cytopathol 2013 121(5):234-41. [Google Scholar]