Breast cancer is one of the most important health concerns of the modern society [1]. Every year, the morbidity of breast cancers in the world were estimated over one million with the mortality rate of over 400 thousand persons [2]. TMX, an antioestrogen molecule and strong hydrophobic drug (water solubility, 0.04 μg/mL at 37°C), is widely administered in breast cancer and high risk patients [3]. Although, TMX was primarily used as a drug against hormone-dependent breast cancers [4], it has also been used in the treatment of hormone insensitive oestrogen receptor negative breast cancers [5,6]. TMX inhibits cell proliferation and induces apoptosis in breast cancer cells such as MCF-7, MDA-MB231 and BT-20 [7,8]. TMX showed anti-tumour effect on breast cancer cells via induction of apoptosis by several mechanisms, such as inhibition of steroid hormone or halter its receptor, inhibit protein kinase C [9] and binding to calmodulin [10]. It is suggested that TMX can prevent cancer in women at high risk for breast cancer development [11,12]. Previously it has been reported that TMX has oestrogenic effect in the liver and bone and anti-oestrogenic effect in the breast [13]. Although the effective dose of TMX has been approved, its side effects are still a therapeutic challenge in postmenopausal women with breast cancer. The adverse effects are polyps, hyperplasia, carcinoma, sarcoma [14,15], vaginal haemorrhage, blazes and liquid retention [16,17] and development of endometrial cancer [18]. According to the reports, there are more side effects to use of TMX; among them are development of liver cancers, increase of blood clotting factor, retinopathy and corneal opacities [19,20]. So, for the long-term chemotherapy of breast cancers the colloidal delivery systems are subjected as the best way to deliver TMX. These are the SLNs, lipospheres, or nanospheres produced by pharmaceutical researches from the early 1990s [21]. In addition, for encapsulation of low-dose TMX in colloidal delivery systems, nanospheres like polycaprolactone were used. The aim of this formulation was to minimize the toxic effects of treatments on normal tissues, and obtain the necessary dose of drug for a known period at tumour location [22]. An in vitro study showed that encapsulation of TMX citrate in polymeric nanoparticles increased its oral bioavailability about three to five fold in comparison to the pure drug [23].
We have previously reported the effect of TMX-loaded SLN on the LA7 cell-induced rat mammary tumour gland. The result showed that TMX-loaded SLN have similar effect on the tumour as free TMX, which promotes apoptosis in the rat mammary gland tumour. The efficacy of free-TMX and TMX-loaded SLNs was similar. However, the TMX-loaded SLN showed a more prolonged effect suggesting that incorporation of TMX in SLN is suitable for delayed drug release in the chemotherapy of breast cancers, while decreasing its hepatotoxic effects [24,25]. Since, there are side effects to the use of TMX, colloidal delivery systems, solid lipid nanoparticles were suggested to delivery of TMX for long-term chemotherapy of breast cancers. However, it is necessary to determine the effect of nanoparticles on the liver and kidney of mammals as two organs are involved in metabolism. Therefore, the objective of the current study was to compare the hepato toxicity and renal toxicity effect of TMX and TMX-loaded SLNs on ovariectomized female Wistar rats.
Materials and Methods
The experimental procedure approved at Hamadan University of Medical Sciences (UMSHA) and the research was conducted according to the guidelines for the care and use of laboratory animals of UMSHA.
Preparation of SLN and TMX-loaded SLN: In the production process of SLN, high-pressure homogenization and soy lecithin was used respectively to incorporate TMX as a lipophilic agent into the matrix of palm oil and the surfactant in SLN dispersion.
SLN was prepared according to the previous report using the High-Pressure Homogenization (HPH) technique [26]. Briefly, 50 mg SLN containing S154 (Softisan®154 or hydrogenated palm oil, CONDEA Witten, Germany) and S100 (soy lecithin, Lipoid KG Ludwigshafen, Germany) with a ratio of 70:30, mixed with a solution containing 1 mL oleyl alcohol (Sigma), 5 mg thimerosal (Sigma), 4.75 gm sorbitol (Sigma), 89.25 mL bidistilled water and TMX (Sigma) with a concentration of 10 mg. The mixture was stirred on a magnetic stirrer and then Ultra Turrax® (Ika, Staufen Germany) at 13000 rpm for 30 minutes at room temperature to obtain the SLN. For planning of TMX-loaded SLN, Ultra Turrax® (Ika, Staufen Germany) at 13000 rpm for 10 minutes was used to prepare the ratio of SLN and TMX (5:1). The mixture of SLN and TMX was incubated at 50°C-60°C while stirring overnight with a teflon coated magnet at 500 rpm. Then TMX-loaded SLN was exposed to air until solidification.
SLN and TMX-loaded SLN characterized using high-performance particle sizer (HPP5001, Malvern Instruments, Worcestershire, UK) for Particle sizes and particle-size distribution (PI). An analyser (Zeta sizer; ZEN 2600, Malvern) in triplicate measured the zeta potential (i.e., electrophoretical movement) of the SLN and TMX-loaded SLN. High performance liquid chromatography (Waters 2695, New Jersey, USA) with UV-VIS detector was used for determination of Drug Loading (DL%) and Entrapment Efficiency (EE%).
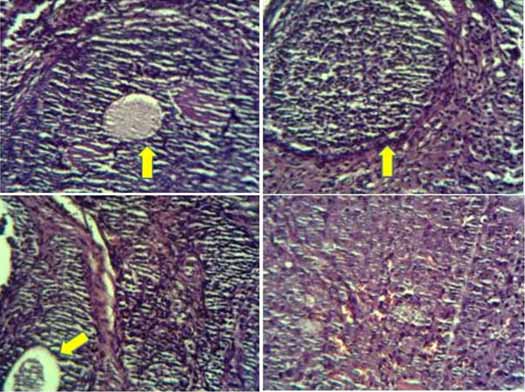
Experimental design and procedure: Twenty-four of six to eight weeks old female Wistar rats with average weight of 150 gm-160 gm were obtained from Pasteur Institute of Iran, Iran. They were housed in standard cage (each cage for two rats), appropriate conditions include cyclic light (12-h light/dark cycles). Chew diet and tap water were available ad libitum throughout the experiment. To remove the variation of oestrogenic effect in the sexual cycles, the animals were ovariectomized before treatment. The rats were anaesthetised with a mixture of ketamine/xylazin (100/5 mg/kg bw) by intraperitoneal injection and were bilaterally ovariectomized under standard method [27]. To confirm the accurateness of ovarian tissue removing, sections of rat’s ovary stained using H&E method and viewed by light microscope [Table/Fig-1]. After operation, the animals were allowed to recover for two weeks before the commencement of the study. Then, the animals were divided into four groups of six rats each. The animals were weighted before starting the treatment. The first group received TMX (2 mg/Kg b.w.) dissolved in 1 mL olive oil, the second group received TMX-loaded SLN (2:10 mg/kg b.w.) dispersed in 1 mL olive oil, the third group comprised untreated ovariectomized and served as the ovariectomized control group (Control) and group 4 served as the healthy control group (Healthy). The Group 3 and Group 4 received 1 mL olive oil and deionized water respectively. They were daily treated orally for 21 consecutive days (by gavage). The responses of the liver and kidney was studied by determining serum ALT, AST, ALP, LDH activities and measurement of serum UA, BUN, Cre and urea. At the end of the study, weight of the rats was recorded and then they were sacrificed and their blood samples were collected by cardiac puncture using 23 G or 26 G needles. The blood samples were allowed to clot at room temperature, centrifuged at 1000 gm for 10 minute, serum was separated and analysed for ALT, AST, ALP, LDH activities and measurement of uric acid UA, BUN and Cre by a Chemistry Analyser (BS, 380, China) using standard diagnostic kits (Pars Azmun, Iran). The serum level of urea was calculated using the equation {Urea (mg/dL) = BUN (mg/dL) * 2.14}. In addition, the ratio of BUN to Cre was determined. The animals were also studied for structural abnormalities in the liver and renal tissues.
Structure of ovary of rats after ovariectomy (H&E, X40) in studied groups showing follicles (arrow).
Histopathological study: At the end of the study, the rats were sacrificed and examined for tissue abnormalities. Samples of liver and kidney from all groups were immediately fixed in 10% formalin overnight, embedded in paraffin, cut into 5 mm sections, placed on slides and stained with Haematoxylin and Eosin (H&E). The tissue sections were viewed under a light microscope (40X magnification) (Leitzdialax20, Germany).
Statistical Analysis
The data were expressed as mean±standard deviation. For statistical analysis, the experimental values were compared to their corresponding control. Statistical analyses were conducted by one-way Analysis of Variance (ANOVA) in SPSS software (Version 16.0) followed by post-hoc test (Tukey HSD) to compare means. The significant difference was considered to be p<0.05 or less.
Results
Characteristics of SLN and TMX-loaded SLN: The average size of TMX-loaded SLNs (251.65±33.02 nm) was significantly (p<0.05) larger than that of the free SLNs (152.87±9.91 nm). The surfaces of SLN and TMX-loaded SLNs carried a negative charge (-15.7±1.12 mV) and positive charge (+10.16±0.2 mV) respectively. The entrapment efficiency and drug loading of TMX-loaded SLN were 89.98±1.5 % and 17.99±1.9 % respectively. Poly dispersity index was 0.22±0.05 for SLN and 0.48±0.11 for TMX-loaded SLN.
Effect of TMX and TMX-loaded SLN on the body weight: The results showed that treatment with TMX and TMX-loaded SLN did not negatively affect the body weighs of these animals [Table/Fig-2]. The body weight of all animals increased during the study period and there were no significant (p>0.05) differences among treatment groups.
Effect of treatment with TMX and TMX-loaded SLN on body weight (BW) of female ovariectomized Wistar rats.
| Groups B.W. | TMX | TMX-SLN | Control | Healthy | p-value |
|---|
| Pre treatment | 166.17±3.31ab* | 165±4.12ab* | 153.50±1.52 | 152±3.19 | 0.00 |
| Post treatment | 258.33±17.57 | 257.00±16.78 | 245.16±41.80 | 227.33±10.11 | 0.142 |
All values are expressed as mean ± standard deviation; groups; TMX=tamoxifen; TMX-SLN=tamoxifen loaded SLN; Pretreatment = Day 0; Post treatment = Day 21.
a comparing with healthy group.
b comparing with control group.
*Means significant; p< 0.001
Effect of TMX and TMX-loaded SLN on the liver enzymes: The effects of treatment with TMX and TMX-loaded SLN on the liver enzymes are shown in [Table/Fig-3]. As shown in the table, although there was not significant difference in level of ALP activities between groups, there was slight increase in ALP and ALT activities and decrease LDH and AST activities (p>0.05) in ovariectomized rats compared to healthy normal rats. TMX and TMX-loaded SLN treatment resulted in insignificantly decrease in ALP, LDH and ALT activity in comparison with ovariectomized control group. According to the data in [Table/Fig-3] the animals treated with TMX showed significant decrease (p<0.05) in AST activity compared to the healthy group. Activity of AST was decreased in TMX-loaded SLN group in comparison to healthy and ovariectomized animals. However, it was not significant.
Effect of treatment with TMX and TMX-loaded SLN on liver enzymes of female ovariectomized Wistar rats.
| Enzymes Groups | ALPU/L | LDHU/L | AST(OT)U/L | ALT(PT)U/L | OT/PTratio |
|---|
| TMX | 569.17±229.10 | 1388±559.12 | 100.83±15.64a* | 46.17±13.64 | 2.30±0.55 |
| TMX-SLN | 717.67±215.36 | 1457±879.09 | 113.67±29.88 | 49.17±6.18 | 2.27±0.37 |
| Control | 735.00±161.80 | 1533±524.67 | 165.33±54.36 | 60.17±18.32 | 2.77±0.36 |
| Healthy | 536.00±258.50 | 1940±891.51 | 174.83±77.13 | 58±21.54 | 3.00±0.74 |
| p value | 0.29 | 0.144 | 0.012 | 0.98 | 0.65 |
All values are expressed as mean ± standard deviation; AST=aspartate amino transaminase; OT=Glutamate Oxaloacetate Transaminase; ALT=alanine amino transaminase; PT=Glutamate Pyruvate Transaminase; ALP=alkaline phosphatase; LDH=lactate dehydrogenase; TMX=tamoxifen; TMX-SLN=tamoxifen loaded SLN
a comparing with healthy group.
Effect of TMX and TMX-loaded SLN on the renal function parameters: The effects of TMX and TMX-loaded SLN on the ovariectomized female rats are presented in [Table/Fig-4]. According to the table there was no significant changes in renal health indicator between groups (p>0.05). Although the operation of ovariectomy resulted in increment of Cre, urea and decrease in BUN compared to the healthy control group, the changes were not significant. There was no significant difference in UA between ovariectomy and healthy animals. Treatment with TMX decreased BUN and increased creatinine and uric acid in comparison to ovariectomized and healthy control groups. Encapsulation of TMX inside the SLN decreased its effect on the creatinine and uric acid but increased its effect on the BUN. The only significant result was shown for decreased ratio of BUN to Cre in TMX group compared to healthy group.
Effect of treatment with TMX and TMX-loaded SLN on renal function parameters of female ovariectomized Wistar rats.
| Parameters Groups | BUNmg/dL | Cremg/dL | Ureamg/dL | UAmg/dL | BUN/Creratio |
|---|
| TMX | 26.00±3.85 | 0.68±0.04 | 55.64±8.23 | 2.1±0.45 | 38.017±4.78a* |
| TMX-SLN | 29.500±3.73 | 0.58±0.26 | 63.13±7.98 | 1.66±0.5 | 44.33±5.00 |
| Control | 27.17±4.31 | 0.67±0.82 | 58.14±9.22 | 2.05±0.53 | 40.60±6.13 |
| Healthy | 29.50±4.42 | 0.62±0.04 | 54.80±24.76 | 2.02±1.06 | 48.30±9.22 |
| p value | 0.189 | 0.61 | 0.75 | 0.19 | 0.049 |
All values are expressed as mean ± standard deviation; BUN=blood urea nitrogen; Cre=creatinine; UA=uric acid; TMX=tamoxifen; TMX-SLN=tamoxifen loaded SLN.
a comparing with healthy group.
*Means significant; p< 0.05
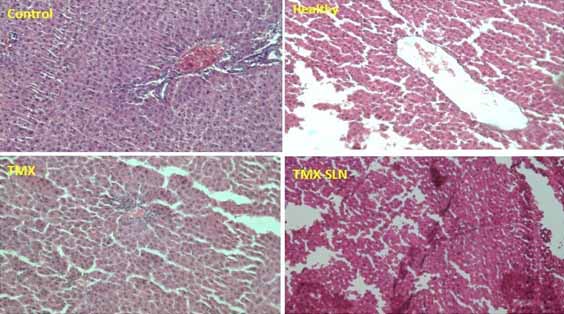
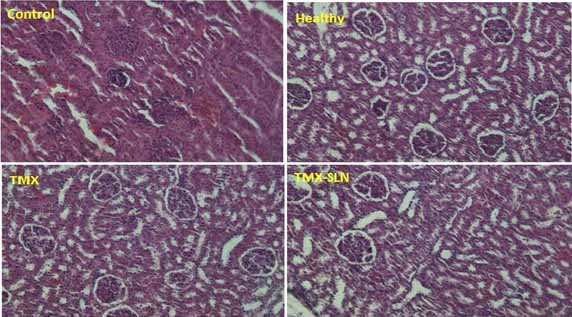
Effect of TMX and TMX-loaded SLN on the histopathology of liver and kidney: Histopathological features of the liver and kidney are shown in [Table/Fig-5,6] respectively. According to the obtained results the liver and kidney of animals under ovariectomy and exposed to TMX and TMX-loaded SLN showed no important tissue injury. Normal and regular structures were observed in all groups.
Histopathological findings of rat’s liver after treatment with TMX and TMX-SLN for 21 days (H&E, X40) compared to Control and Healthy animals.
Histopathological findings of rat’s kidney after treatment with TMX and TMX-SLN for 21 days (H&E, X40) compared to Control and Healthy animals.
Discussion
In comparison of the average size, TMX-loaded SLNs was significantly larger than free SLNs (251.65±33.02 versus 152.87±9.91 respectively). The charge of TMX-loaded SLNs was positive (+10.16±0.22). This positive charge was related to the incorporation of drug in particle surface or entangled in aliphatic chains of triglycerides [20]. One of the important parameter for nanoparticles worth is particle size due to its considerable impact in the biological tests. When TMX was incorporated into SLNs, the increase in particle size suggested that loaded TMX was either adsorbed onto the particle surface or entangled in the aliphatic chains of triglycerides.
As an important factor, Zeta potential could be helpful to determine the stability of colloidal systems. TMX in corporation with nanoparticle could neutralize the negative charge of the complex even to the positive potential. This changing of charges may be due to amino group of TMX and its localisation on the surface of SLNs [18].
Previous studies have shown the effect of estrogen therapy on preservation of bone mineral density in postmenopause women [28]. While the results of some study showed a non-significant increase of ALP activity in ovariectomized rats as compared to the healthy control group [29], others reported reduced serum alkaline phosphatase level in the postmenopausal group as compared to women in the premenopausal period [28]. There are some reports based on increased serum ALP level in women with breast cancer and the effective role of TMX on its reduction [30]. Although in another research, no significant difference was observed in ALP level between the control and groups treated with TMX after a period of three months [11].
Based on the results presented in [Table/Fig-3]; treatment of ovariectomized rat with free TMX compared to TMX-loaded SLN resulted in further reduction in serum ALP activity; this may be due to the slow release of the drug from the SLNs. However, TMX encapsulated in SLN decreased ALP activity as compared with the ovariectomized control group. The biochemical markers of bone turnover such as APT are not used for determination of osteoporosis or bone density except they are considered for predicting disease management. It is believed that postmenopausal women take oestrogen for at least seven years to maintain bone mineral density [28].
Blood biochemical parameters can be useful in the diagnosis of toxicity because they can be easily conducted in most diagnostic laboratories. Liver function can be determined by study of serological markers such as alanine amino transaminase, ALT [31]. The results of this study showed a non-significant decrease in serum AST activity in ovariectomized rat, as compared to the healthy control group and this may be due to the early response of the liver to lack of estrogen. Treatment with TMX reduced significantly activity of AST as compared to the healthy control group. However, TMX insignificantly reduced AST activity compared to ovariectomized control group. Also, the significant difference between ovariectomized control and TMX-loaded SLN groups was not shown. Since, there was no significant result between two groups; TMX and TMX-loaded SLN, suggesting that TMX-loaded SLN maintains the biological properties of TMX. In a similar study, the effect of TMX on the serum of ovariectomized rats at 2 mg/kg/24 hour, statistically showed that there was no significant change in liver enzymes such as AST and ALT [13]. In another study, TMX-encapsulated Nanoparticles (NPs) were used to treat tumour-induced rats. The results showed more hepatic toxicity and renal toxicity of free TMX in comparison to TMX-NPs. In this study, TMX and TMX-NPs reduced serum AST and ALT as compared to the control group [32].
According to [Table/Fig-3], although ovariectomy increased serum ALT, this was not statistically significant and may be due to the short duration of the study. It agreed with a study which showed significant elevated plasma ALT as markers of acute or chronic liver injury, after four months of rat ovariectomy [33]. In the present study, treatment with TMX and TMX-SLN caused a non-significant decrease in ALP activity as compared to the healthy and ovariectomized groups. The earlier study which used 2 mg/kg/24 hour did not find any significant difference in serum ALT activity between the groups (ovariectomized and sham groups) [34].
The results of the current study showed the impact of ovariectomy as the insignificant reduction of LDH activity, same as other reports which showed a slow decrease of LDH activity following ovariectomy [35-37]. It can be concluded that by exposing the tissue to oxygen tension, LDH activity may be modified by ovarian hormones [35]. According to the results obtained, treatment with TMX and TMX-loaded SLN further reduced LDH activity, although it was insignificant as compared to the healthy control and ovariectomized groups. These results are concurrent with other studies that reported a reduced LDH activity in ovariectomized animal treated with TMX [38] and mastectomy in women who were treated with 10 mg TMX per day [39]. In another study, the activity of LDH enzyme was significantly reduced when healthy mice were treated with TMX. However, in this study when TMX was combined with vitamin C, no significant difference was observed in all the glycolytic enzymes (LDH, HK and PK) of the experimental and control groups. Although the mechanism of the effect of TMX is not so clear, it is possible that TMX switches between aerobic and anaerobic pathways and inhibits LDH activity [12].
Renal disorders can be determined by serological markers such as urea, BUN, Cre and UA. The results of serum analysis showed a decreased BUN and BUN to Cre ratio after ovariectomy, although they were not significant. While in the present study, ovariectomy had no effect on serum creatinine and uric acid, a previous study have reported a decrease in uric acid following ovariectomy [40]. Treatment with free TMX further showed insignificant decrease in BUN and significant fall in BUN to creatinine ratio in comparison to the healthy and ovariectomized groups. Although TMX-loaded SLN increased the level of BUN, urea and BUN to creatinine ratio in comparison to the ovariectomized group, the variation was not significant. In the TMX treated group, serum levels of Cre and UA remained unchanged as compared to the ovariectomized control group but TMX-loaded SLN caused a negligible reduction in creatinine and uric acid level in comparison to the ovariectomized control and healthy groups. Therefore, when TMX was encapsulated inside the SLN formulation, it moderately improved the kidney because its release was sustained. Similar results were reported by Pandey SK et al., that less hepatotoxicity and renal toxicity were raised from TMX-loaded NPs as compared to the free TMX in the evaluation of AST, ALT activities and plasma levels of BUN, creatinine and urea [32]. In another study, it was reported that when TMX and vitamin C were administered to normal healthy mice, the result showed no side effect on the level of liver function enzymes. The study also showed that, there was an insignificant difference in the level of renal biomarkers such as UA, urea and Cre [12]. A previous study showed the less effect of TMX-loaded SLN on the liver enzymes of Sprague-Dawley rats with induced mammary gland tumour [24].
In the current study, the histopathological results showed no significant tissue structural abnormalities which may be due to the short duration of the study. This result confirmed that the biological availability of drug is not affected when incorporated into SLN. Therefore, SLN could be applied as a drug delivery system for cancer treatments. When TMX encapsulated inside SLN, because of its small size, could not be easily phagocytosed by macrophages and therefore the NPs could be potentially used in long-term circulating carrier system for breast cancer therapy. The SLN formulation will help to increase the solubility of the drug and facilitate the entrapment of high amounts of drug in the NPs [6]. It was previously reported that loading of TMX inside SLN enhanced the treatment efficacy of TMX [24]. As a result, TMX encapsulated inside the SLN, has similar effect on the liver and kidney as free TMX. More studies could be useful to evaluate the efficacy of TMX-loaded NPs in postmenopausal women.
Limitation
The evaluation of all serum biochemical parameters including the proteins, enzymes, cytokines and hormones could be useful in determining the mechanism of variations in serum biochemical parameters raised from TMX and TMX-loaded SLN.
Conclusion
The current findings indicated that there is no statistically significant difference between the effect of TMX and TMX-loaded SLN on the liver and kidney of ovariectomized rats. Therefore, when TMX loaded inside the SLN, its biological activity is still preserved, suggesting that SLN is a good carrier for the drug insoluble in water. In addition, the TMX-loaded SLN, because of its small size, could not be easily phagocytized by macrophages and therefore the nanoparticles could be potentially used in long-term circulating carrier system for breast cancer therapy. However, further studies are warranted to optimise and to develop more, this kind of drug delivery system in the treatment of cancers. Yet, further research using clinical trials will be needed to determine if the results obtained in this study can be extrapolated to humans.
Financial support: The study was funded by Vice-chancellor for Research and Technology, Hamadan University of Medical Sciences (No. 940201314).
All values are expressed as mean ± standard deviation; groups; TMX=tamoxifen; TMX-SLN=tamoxifen loaded SLN; Pretreatment = Day 0; Post treatment = Day 21.
a comparing with healthy group.
b comparing with control group.
*Means significant; p< 0.001
All values are expressed as mean ± standard deviation; AST=aspartate amino transaminase; OT=Glutamate Oxaloacetate Transaminase; ALT=alanine amino transaminase; PT=Glutamate Pyruvate Transaminase; ALP=alkaline phosphatase; LDH=lactate dehydrogenase; TMX=tamoxifen; TMX-SLN=tamoxifen loaded SLN
a comparing with healthy group.
All values are expressed as mean ± standard deviation; BUN=blood urea nitrogen; Cre=creatinine; UA=uric acid; TMX=tamoxifen; TMX-SLN=tamoxifen loaded SLN.
a comparing with healthy group.
*Means significant; p< 0.05