Squamous cell carcinoma of the lower lip is a malignant neoplasm of epithelial origin. Most cases occur in white men above the fourth decade of life and chronic sun exposure is considered to be the main etiologic factor [1,2]. Generally, this tumour presents indolent biological behaviour, with low rates of recurrence and metastasis; however, it is associated with aesthetic and psychological morbidities [3,4].
In the tumour microenvironment, malignant cells can be detected and eliminated by the immune system, a phenomenon described as "immune surveillance" [5]. NK cells and cytotoxic T lymphocytes (CD8+) stand out for their ability to recognize tumour cells and destroy them through cytotoxic effects mediated by perforin and granzymes [6].
NK cells recognize tumour cells with reduced or absent expression of class I molecules of the major histocompatibility complex (MHC class-I). Therefore, they are considered the first line of defense against tumours [7]. In the advanced stages of maturation, they express CD57, a biomarker that has been used to assess the immune status of patients with cancer [8-10]. In contrast to CD57+ cells, CD8+ T lymphocytes are involved in adaptive immune responses and their activity is restricted to recognition of antigenic peptides associated with MHC class-I [5,11].
In recent studies, the densities of CD57+ and CD8+ cells in the tumour microenvironment were found to be associated with the prognosis of malignancies, such as melanoma and colorectal carcinoma. In general, the high expression of these cells was associated with a less aggressive clinical course and improved survival [12-15].
Understanding the anti-tumour immunity is important since it may contribute to the identification of prognostic markers and development of new therapies. Therefore, the objective of this study was to evaluate the immunoexpression of CD57+ and CD8+ cells in lower lip SCCs, in order to assess possible correlations with clinico-pathologic parameters.
Materials and Methods
A retrospective study was conducted at the Dr. Luiz Antônio Hospital, Natal, Rio Grande do Norte (RN), Brazil, in a two-year period, from 2012 to 2014. Thirty-two lower lip SCCs specimens were retrieved and divided into two groups: a group consisting of patients with nodal metastasis at diagnosis and a nonmetastatic group. Only patients undergoing surgical resection without prior radiotherapy or chemotherapy, and a minimum follow up of five years were included. Cases with incomplete medical records and insufficient biological material for histopathological and immunohistochemical analysis were excluded.
Clinical data including Tumour-Node-Metastasis (TNM) staging and presence or absence of nodal metastasis were collected from medical records. This study was approved by the Research Ethics Committee of the Federal University of Rio Grande do Norte, Brazil (protocol n° 508.462) and was in accordance with the Helsinki Declaration.
Morphologic analysis: For the morphologic analysis, sections (5 μm) were obtained from paraffin-embedded specimens and stained with haematoxylin/eosin (H/E). Two previously calibrated and independent observers established the histologic grade of malignancy at the tumour-host interface [16] in a blinded manner. Parameters analysed included: degree of keratinization, nuclear pleomorphism, pattern of invasion, and inflammatory infiltrate. Semiquantitative scores from 1 to 4 were attributed for each parameter, and then summed up [16]. According to the final score the cases were classified as low-grade malignancy (score ≤8) or high-grade malignancy (score >8) [17]. Occasional disagreements were discussed to reach a consensus.
Immunohistochemical methods: Sections (3 μm) were cut from paraffin-embedded tissue blocks and mounted on glass slides with organosilane (3-aminopropyl) triethoxysilane; Sigma-Chemical Co., St Louis, MO, USA). Histologic sections were deparaffinized, rehydrated and subjected to antigen retrieval in Trilogy solution (Cell Marque, CA, USA). The samples were immersed in 3% hydrogen peroxide to block endogenous peroxidase activity. The antibodies against CD57 (clone TB01, Dako, Glostrup, Denmark) and CD8 (clone C8/144B, Dako, Glostrup, Denmark) were diluted at 1:100 and 1:500 respectively, and samples were incubated for 60 minutes. The sections were then washed twice in Phosphate Buffer Solution (PBS) and treated with streptavidin-biotin peroxidase complex (LSAB+System-HRP; Dako, Carpinteria, CA, USA) at room temperature. Peroxidase activity was developed by immersing tissue sections in diaminobenzidine (Liquid DAB+substrate; Dako), resulting in a brown reaction product. Finally, sections were counterstained with Mayer’s haematoxylin and cover slipped. Sections of lymph nodes served as positive controls. Negative controls were obtained by omitting the primary antibodies.
Immunohistochemical assessment: All immunostained slides were scanned using Panoramic 3DHISTECH MIDI equipment (3DHISTECH Ltd., Hungary). Five random microscopic fields at the invasion front were photographed (Scale bar =100 μm) using microscopy software Panoramic Viewer, version 1.15.2 (Copyright 2013 3DHISTECH Ltd., Hungary). CD57+ and CD8+ cells were analysed quantitatively by a single trained examiner unaware of clinico-pathologic data. The counts were performed within an area of 500 μm from the tumour border. For each case, the mean number of CD57+ and CD8+ cells was determined per microscopic field (20X according to the software specifications), using an adaptation of the method described by Zancope E et al., [18].
Statistical Analysis
The results were analysed using Statistical Package for Social Sciences, version 20.0 (SPSS, Chicago, IL, USA). Non-parametric Mann-Whitney (U) test was performed to assess possible differences between CD57+ and CD8+ cell counts according to clinical and morphologic variables. Spearman’s correlation coefficient (r) was applied in order to evaluate the correlation between immunoexpressions. A p<0.05 was considered statistically significant.
Results
Clinical and morphologic analysis: Thirty-two patients of lower lip SCC were included in this study. The male/female ratio was 1.9/1 and the mean age was 68.7±16.2 years. With respect to TNM staging, 17 (53.1%) patients were in stages III/IV and 15 (46.9%) in stages I/II. Concerning nodal metastasis, 16 (50%) cases were classified as low-grade and 16 (50%) as high-grade. Local recurrence occurred in 3 (9.4%) cases and death in 5 (15.6%) cases. Most patients in stages III/IV had high-grade malignancy tumours (70.6%) (p=0.032). The presence of nodal metastasis was significantly associated with high grade malignancy tumours (p=0.006) [Table/Fig-1].
Histologic grade of lower lip squamous cell carcinoma according to nodal metastasis and clinical stage.
| Variables | Histologic grade | Total | p-value |
|---|
| High graden (%) | Low graden (%) | n |
|---|
| Nodal metastasis |
| Absent | 4 | 12 | 16 | 0.006 |
| Present | 12 | 4 | 16 |
| Clinical stage |
| Stages I/ II | 4 | 11 | 15 | 0.032 |
| Stages III/ IV | 12 | 5 | 17 |
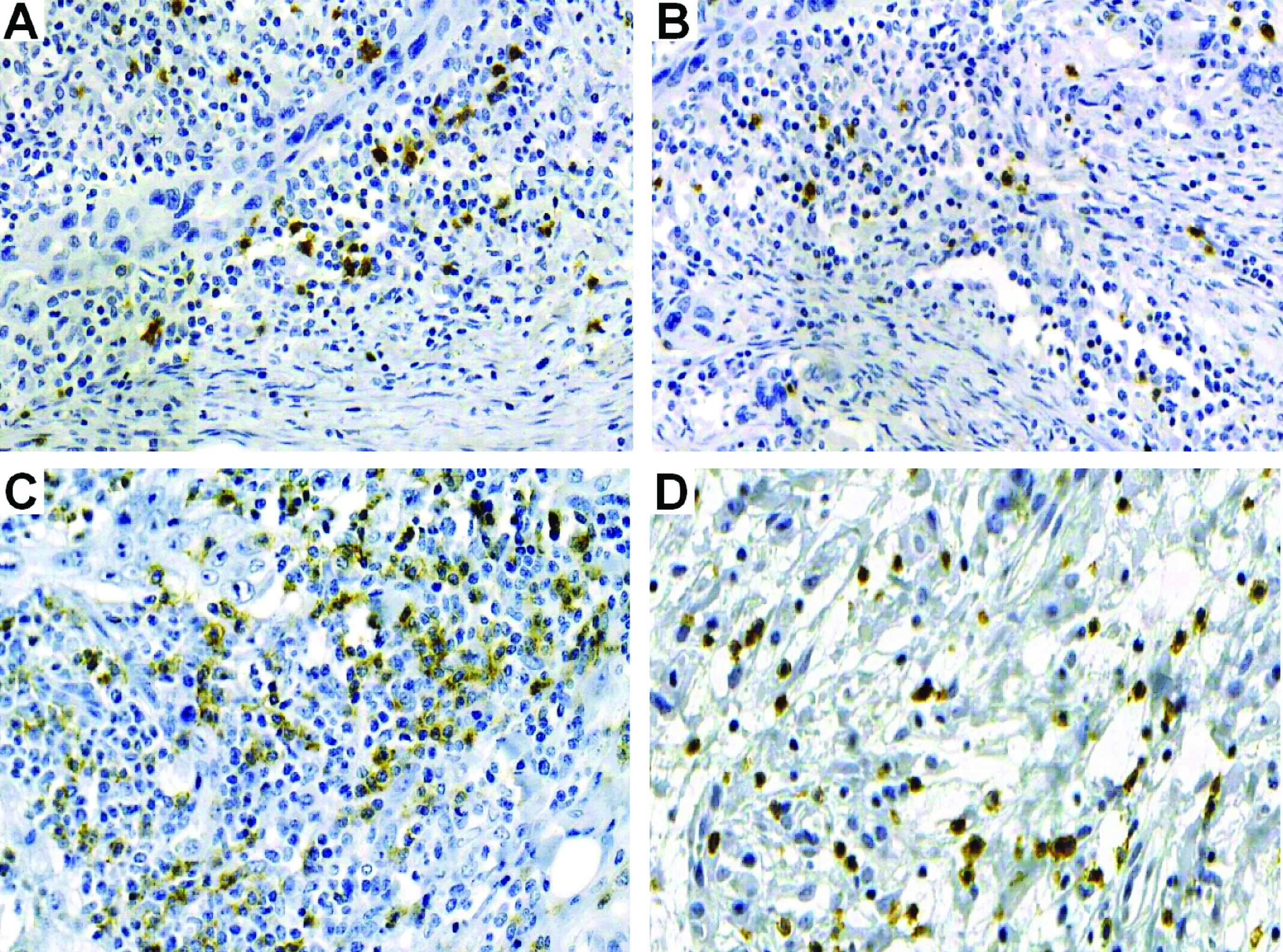
Immunohistochemical analysis: All cases were positive for CD57 and CD8 antibodies, which could be evidenced by a membrane and cytoplasmic staining, regardless of intensity [Table/Fig-2]. For the nonmetastatic tumours, CD57+ and CD8+ cell counts in invasion front were found to be higher when compared to the metastatic cases, although, not statistically significantly (p>0.05) [Table/Fig-3,4]. Similarly, a higher concentration of CD57+ cells and CD8+ T lymphocytes was observed for early-stage tumours (stages I/II) compared to advanced-stage tumours (stages III/IV), as well as for low-grade lesions compared to high-grade lesions, but these differences were not significant (p>0.05) [Table/Fig-3,4].
Immunohistochemical staining. a) NK cells (CD57+) in the invasion front of nonmetastatic and b) metastatic lower lip SCC; c) CD8+ T lymphocytes in the invasion front of nonmetastatic; d) metastatic lower lip SCC (SABC, 20X).
Parameters used for the calculation of Mann–Whitney (U) test for the evaluation of NK (CD57+) cell counts according to the clinico-pathologic variables.
| Variables | n (%) | Median | Q25-Q75 | Mean of ranks | U | p-value |
|---|
| Nodal metastasis |
| Absent | 16 (50.0) | 156.5 | 76.9-209.2 | 17.5 | 111.5 | 0.622 |
| Present | 16 (50.0) | 140.7 | 78.3-163.5 | 15.4 |
| Clinical stage |
| Stages I/ II | 15 (46.9) | 167.0 | 76.4-213.0 | 17.5 | 112.5 | 0.576 |
| Stages III/ IV | 17 (53.1) | 137.4 | 82.3-175.5 | 15.6 |
| Histologic grade |
| Low grade | 16 (50.0) | 158.90 | 81.5-209.2 | 18.0 | 102.5 | 0.936 |
| High grade | 16 (50.0) | 136.40 | 67.2-163.5 | 14.9 |
| Local recurrence |
| Negative | 29 (90.6) | 146.4 | 83.3-205.4 | 17.2 | 21.0 | 0.146 |
| Positive | 3 (9.4) | 78.6 | 21.4-111.3 | 9.0 |
| Clinical outcome |
| Remission | 27 (84.4) | 137.4 | 76.4-213.0 | 16.6 | 64.0 | 0.856 |
| Death | 5 (15.6) | 150.4 | 86.9-160.1 | 15.8 |
Parameters used for the calculation of Mann–Whitney (U) test for the evaluation of CD8+ T cell counts according to clinical-pathologic variables.
| Variables | n (%) | Median | Q25-Q75 | Mean of ranks | U | p-value |
|---|
| Nodal metastasis |
| Absent | 16 (50.0) | 140.7 | 70.3-246.6 | 18.0 | 103.0 | 0.346 |
| Present | 16 (50.0) | 115.3 | 84.5-183.1 | 14.9 |
| Clinical stage |
| Stages I/ II | 15 (46.9) | 192.4 | 63.4-250.4 | 19.0 | 89.0 | 0.146 |
| Stages III/ IV | 17 (53.1) | 115.2 | 84.8-144.1 | 14.2 |
| Histologic grade |
| Low grade | 16 (50.0) | 127.1 | 95.5-246.6 | 18.0 | 102.5 | 0.936 |
| High grade | 16 (50.0) | 117.4 | 59.2-183.1 | 14.9 |
| Local recurrence |
| Negative | 29 (90.6) | 131.6 | 88.3-225.2 | 17.4 | 16.0 | 0.075 |
| Positive | 3 (9.4) | 38.2 | 26.0-79.4 | 7.3 |
| Disease outcome |
| Remission | 27 (84.4) | 120.6 | 84.2-213.6 | 16.5 | 65.0 | 0.897 |
| Death | 5 (15.6) | 115.2 | 69.6-232.9 | 16.0 |
The cases of lower lip SCC that developed local recurrence exhibited lower immunoexpression of CD57+ and CD8+ cells compared to those without relapses, although statistical analysis revealed no significant differences (p>0.05) [Table/Fig-3,4]. In addition, no statistically significant difference was observed for counts of CD57+ cells and CD8+ lymphocytes regarding clinical outcome (p>0.05) [Table/Fig-3,4].
Spearman coefficient (r) showed a strong positive correlation, statistically significant, between CD57+ cells and CD8+ T lymphocytes (r=0.761; p<0.001).
Discussion
When compared with intraoral carcinomas, lower lip SCCs has a better prognosis. Five-year survival rate ranges from 80% to 90% if diagnosed in early stages [19,20]. However, when patients exhibit regional lymph node metastasis at diagnosis, survival rate decreases to 25-50% [20]. Primary tumour size, histologic grading, nodal metastasis and locoregional recurrences are some of the main factors related to the prognosis of lower lip SCC [1]. In this study, high-grade malignancy was significantly associated with advanced clinical stages and nodal metastasis. The literature reveals conflicting findings regarding the association between histologic grading of malignancy and tumour biological behaviour [21-23]. Therefore, researches have been conducted to identify biomarkers that may help to predict prognosis of this neoplasm [7,18,24,25].
In this context, several studies have investigated the interaction between the immune response and progression of malignant neoplasms, since an effective immunosurveillance is necessary for preventing the onset and progression of cancer [9,11,26-28]. NK cells and CD8+ T lymphocytes are constituents of the immune system and are the most likely cells to be associated with an effective anti-tumour response.
With respect to the role of NK (CD57+) cells against neoplastic cells, studies have reported an inverse correlation with nodal metastasis in carcinomas of the prostate [10], stomach [27], and vulva [29]. Regarding oral SCC, Türkseven MR and Oygür T [7] found an increased amount of CD57+ cells in early-stage cases in relation to those in advanced stages, as well as a greater number of these cells in low-grade malignancy specimens in comparison to high-grade specimens. Furthermore, Zancope E et al., reported that CD57+ cell counts were higher in nonmetastatic lower lip SCC in comparison with normal mucosa, premalignant lesions and intraoral SCCs [18].
The present study did not show an association between CD57+ cells and clinico-pathologic parameters of lower lip SCC. Nevertheless, in general, these cells were more numerous in less aggressive cases, corroborating previous findings [7,18]. According to some authors [25,30], although CD57+ cells participate in the control of tumour growth, a number of factors are responsible for the suppression of their activity in human tumours, such as increased fas ligand expression, loss of mRNA for granzyme B, subregulation of CD16 protein and the associated ζ chain. This may partially explain the lower expression of CD57+ cells in the lymphocytic infiltrate of advanced tumours.
Mlecnik B et al., reported a significant association between CD8+ lymphocytes and early stages of colorectal cancer [31]. Moreover, Watanabe Y et al., found a lower expression of these cells in intraoral SCCs of worst clinical outcome [32]. In the present study, a higher concentration of CD8+ T lymphocytes was observed in early stage tumours (stages I/II) compared to advanced stage tumours (stages III/IV), but no significant difference was found between the number of these cells and clinical staging. These findings, when taken together, suggest a greater contribution of immune anti-tumour effector cells in early-stage tumours. Additionally, in advanced-stage cases, there could be malfunctions of these cells or failure in their recruitment to the tumour microenvironment.
With regard to the correlation between CD8+ T lymphocytes and histologic grading, a previous study [24] showed a significant higher median of these cells in low-grade malignant oral SCCs. In our research, as in that by Piva MR et al., [11] and Zancope E et al., there was no significant difference between these variables [11,18]. These discordant results could reflect different methods in the quantification of CD8+ T lymphocytes and variations in the studied samples. Additionally, other T-cell subpopulations have been reported to be associated with a more favourable clinical course in cancer, such as Th17 and regulatory T cells (FoxP3+) [31,33]. Thus, the lack of association may be due to the anti-tumour activity of other lymphocyte subpopulations apart from CD8+ T lymphocytes.
In the present study, CD57+ and CD8+ cell counts were not associated with local recurrence and clinical outcome. This may be explained in part by peculiarities inherent to the anatomical location of lower lip SCCs, which provides better visibility for diagnosis and adequate surgical resection than intraoral SCCs [34]. Furthermore, some studies have shown that lower lip SCCs present more CD57+ and CD8+ T cells than oral cavity SCCs [17,18], suggesting that the more indolent behaviour of lip tumours might be due to a more effective anti-tumour immune response and to different characteristics of the anatomical sites [22].
Limitation
A limitation of this study was the sample size, which was based on the availability of archived cases. Furthermore, although immunohistochemistry allows the identification of immune cells, it does not provide information regarding the function of these cells. Therefore, additional studies should be conducted to clarify the influence of CD57+ cells and CD8+ lymphocytes in the biological behavior of lower lip SCC.
Conclusion
Based on the results of the previous and current study, it is possible to suggest that CD57+ cells and CD8+ T lymphocytes participate in the cytotoxic local immune response of the lower lip SCC. Nevertheless, they may not be directly associated with progression and prognosis of the studied lesion. This finding may partially reflect intrinsic aspects of lower lip SCC, which tends to be diagnosed and treated at early stages, enabling a more favourable outcome.