Homozygous Protein C Deficiency in a Premature Infant- A Case Report
Ravi Teja Juloori1, Febe Renjitha Suman2, Rithika Rajendran3, B Uma Maheswari4
1 Postgraduate, Department of Pathology, Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu, India.
2 Professor, Department of Pathology, Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu, India.
3 Senior Research Fellow, Department of Pathology, Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu, India.
4 Professor, Department of Neonatology, Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Febe Renjitha Suman, Professor, Department of Pathology, Sri Ramachandra Medical College and Research Institute, Chennai-600116, Tamil Nadu, India.
E-mail: febemd@gmail.com
Homozygous protein C deficiency is a rare autosomal recessive inherited disorder manifesting as neonatal purpura fulminans. A two-day-old male baby delivered at 31 weeks developed purpuric lesions over the left medial malleolus progressing to other areas. Investigations done showed no detectable protein C activity. Genetic testing identified a homozygous mutation at PROC gene Exon 7; p.Arg211Gln. The diagnosis of inherited homozygous protein C deficiency was made. Heterozygous mutation was identified in the parents. Genetic analysis to detect the heterozygous state in the parents will help in prenatal diagnosis in future pregnancies and in genetic counseling.
Neonatal purpura fulminans, Thrombophilia, Thrombosis
Case Report
We report a two-day-old male baby who developed purpuric lesions after 35 hours of birth over the left medial malleolus during his NICU stay due to prematurity. The child was delivered by emergency lower segment caesarean section in view of foetal distress and breech presentation at 31 weeks of gestation. APGAR scores at birth were 7/10, 8/10 at one minute and five minutes, respectively. Birth weight was 1100 gm. Head circumference and length was in the third to tenth percentile. There were no congenital deformities. Systemic examination was normal. Prophylactic parenteral vitamin K 1 mg was given. This child was born as second in the family out of second-degree consanguineous marriage.
The older sibling diagnosed with Pierre Robin syndrome had died on day 15 of life due to respiratory distress. On day 0, the baby had grunting and retractions and was put on Continuous Positive Airway Pressure (CPAP) support. Clinical examination and laboratory septic workup were found to be normal. On day 2 of life infant developed purpuric rash over the left lower limb medial malleolus and subsequently over the dorsum of the left hand [Table/Fig-1] for which septic workup which included Complete Blood Count (CBC), C reactive protein, procalcitonin and blood culture was done. Haemolytic workup with reticulocyte count, Coombs test and coagulation screen were done.
Purpuric and haemorrhagic lesion over the left medial malleolus, dorsum of left hand.
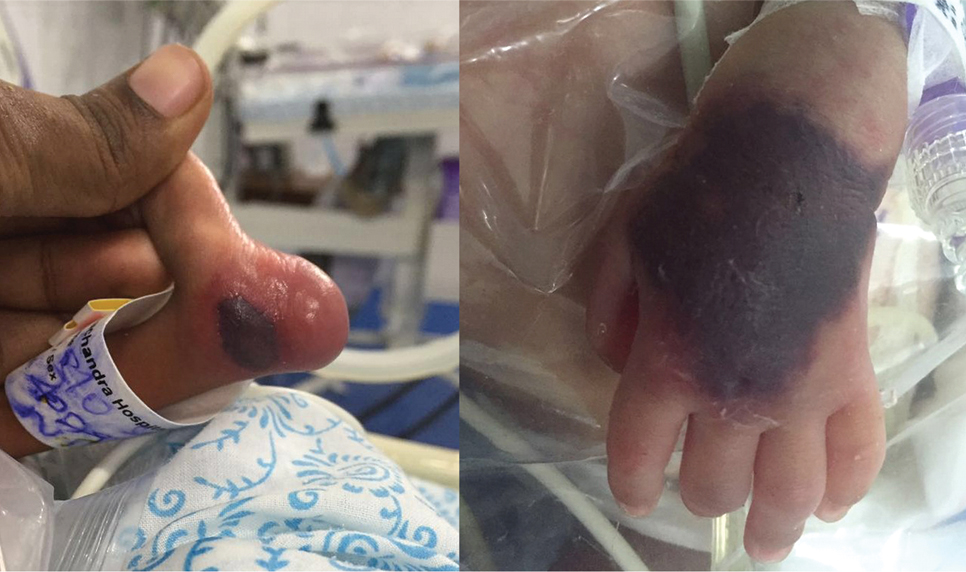
The investigations revealed normal haemoglobin value and leucocyte counts, thrombocytopenia (90,000/μL) prolonged Prothrombin Time (PT) (19.7 seconds), prolonged activated Partial Thromboplastin Time (aPTT) (55 seconds) with low fibrinogen (92.7 mg/dL) and elevated D-dimer levels (2.1 μg/mL). Plasma protein C levels were undetectable and the activity of protein S was 51.5%. Protein C levels were evaluated using the Siemens Berichrom protein C chromogenic assay using Sysmex CS 2400 coagulation analyser. Ultrasound of the cranium and abdomen were normal.
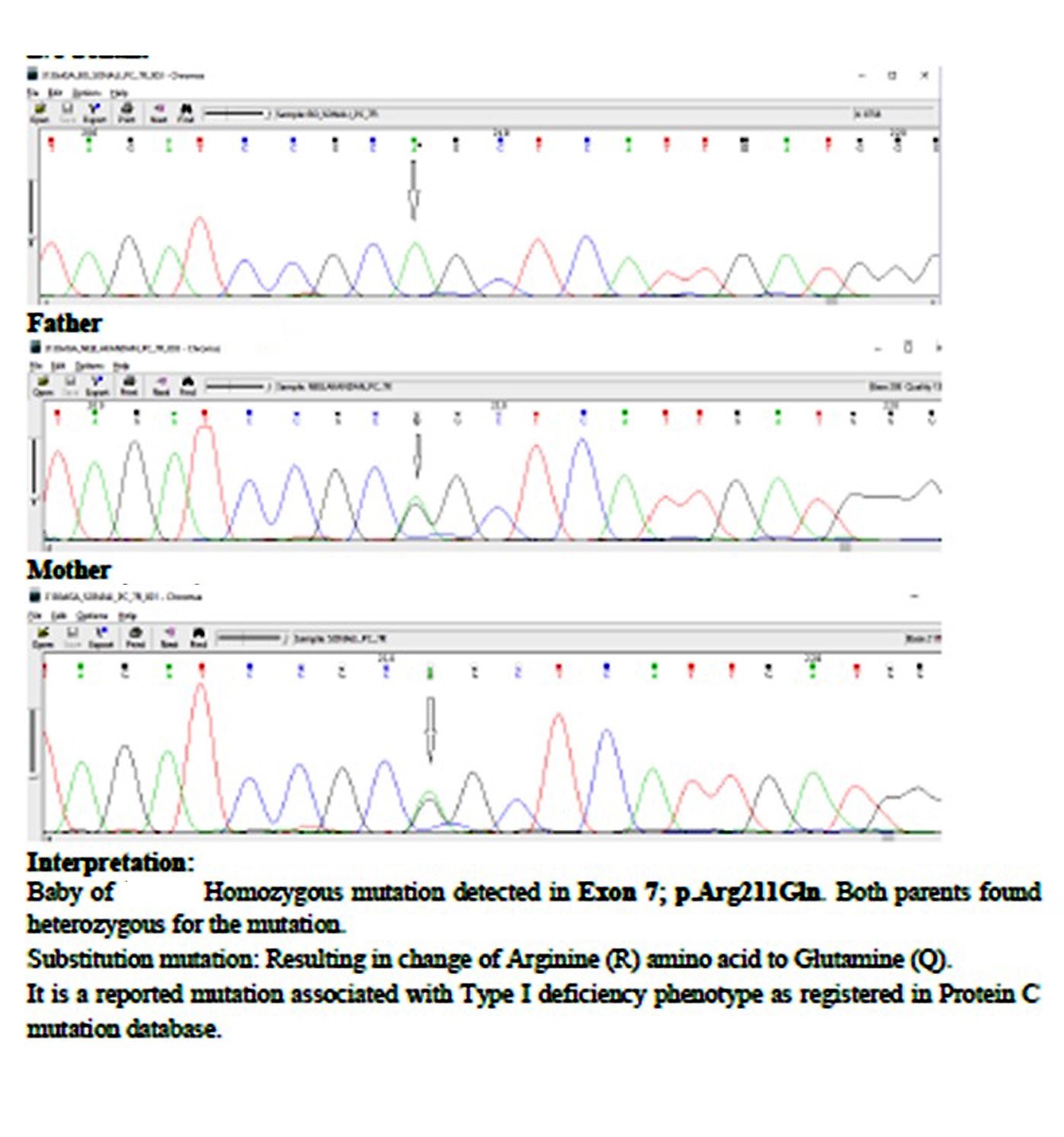
The baby was transfused with platelets, Fresh Frozen Plasma (FFP) and cryoprecipitate. The lesions over the medial malleolus initially subsided but, inspite of the regular 12 hourly transfusion support, the haemorrhagic lesions advanced in other areas including the eye which showed vitreoretinal haemorrhage. The baby was under intensive care and maintained with blood products till 17th day of life and shifted elsewhere for logistic reasons. It was found later on that the baby succumbed due to extensive purpura fulminans. Genetic analysis using Sanger sequencing (NIIH Mumbai) done for the child showed homozygous mutation in PROC gene Exon 7; p.Arg211Gln and the parents had a heterozygous mutation of the same [Table/Fig-2].
Mutational analysis by Sanger sequencing.
This case report was presented after obtaining a written informed consent from the mother to publish as a case report with pictures.
Discussion
Hereditary protein C deficiency is a common cause of neonatal purpura fulminans. Neonatal purpura fulminans first described in 1962 was found to be associated with protein C deficiency in 1983 [1-3]. The congenital protein C deficiency is a rare autosomal disorder with an incidence of 1 per 5,00,000 [4]. Protein C is a vitamin K dependant coagulation protein that is synthesised in the liver and levels are physiologically reduced in the newborn. Protein C deficiency is inherited as autosomal dominant and rarely as autosomal recessive. It is clinically manifested by acute disseminated intravascular coagulation and haemorrhagic necrosis of the skin as observed in this case. Protein C is activated by thrombin and thrombomodulin complex. Activated protein C inactivates factor Va and VIIIa. Hence, protein C deficiency predisposes to thrombin generation and hypercoagulable state [5].
The Protein C gene, PROC, OMIM # 176860, is located in the chromosome 2q 13-14 region and about 230 mutations have been identified [6]. An identical mutation on both alleles leads to autosomal recessive homozygous state and non-identical mutations cause a compound heterozygous condition [4]. This was the case of homozygous protein C deficiency.
A patient similar to the current case was reported in a second-degree consanguineous marriage but with a different molecular defect [7]. The diagnosis of homozygous protein C deficiency is based on the clinical findings, undetectable levels of protein C, a heterozygous state in the parents and identification of the homozygous genetic defect in the infant [8]. Protein C functional (activity) assay was done as it is recommended for initial screening [9]. Preterm infants have protein C levels at 7%-18% of normal [10,11]. At term the protein C levels are approximately 20%-40% of normal levels in the adults which is 78%-118% functional activity [11]. Though, variation seen in the protein C levels of normal neonate makes it difficult to diagnose heterozygous protein C deficiency, homozygous protein C deficiency is easily diagnosed as protein C levels are often undetectable at presentation [12].
Protein C levels may be reduced in other conditions like liver disease, meningococcal infection, uraemia and vitamin K deficiency. Clinical and laboratory features diagnostic of these conditions would be present. Transient reduction in the protein C level is seen in these conditions which is usually over 55% of normal value [13]. The principle of treatment is to control Disseminated Intravascular Coagulation (DIC), inhibit thrombosis and replace protein C. Cryoprecipitate, FFP and platelet transfusions were given to control DIC. FFP was transfused as the source of protein C as protein C concentrate was not available [14,15]. Liver transplant is the curative option which was not done in this case.
Genetic studies and counseling carry an important role in cases of homozygous protein C deficiency especially in consanguineous marriage. Since, this case was born out of second degree consanguineous marriage, many heterozygous members may exist in the pedigree.
Conclusion
Rare cases, like the neonate presented to us showed homozygous inheritance pattern with low to absent protein C activity resulting in neonatal purpura fulminans. Proper genetic counseling and testing are essential so that women at risk can be offered prenatal diagnosis by chorionic villous sampling or foetal blood sampling to prevent the devastating consequences of severe protein C deficiency.
[1]. Van der Horst RL, Purpura fulminans in a newborn babyArch Dis Child 1962 37(194):436 [Google Scholar]
[2]. Sills RH, Marlar RA, Montgomery RR, Deshpande GN, Humbert JR, Severe homozygous protein C deficiencyJ Pediatr 1984 105(3):409-13. [Google Scholar]
[3]. Branson HE, Katz J, Marble R, Griffin JH, Inherited protein C deficiency and coumarin-responsive chronic relapsing purpura fulminans in a newborn infantLancet Lond Engl 1983 2(8360):1165-68. [Google Scholar]
[4]. Lane DA, Mannucci PM, Bauer KA, Bertina RM, Bochkov NP, Boulyjenkov V, Inherited thrombophilia: Part 2Thromb Haemost 1996 76(6):824-34. [Google Scholar]
[5]. Price VE, Ledingham DL, Krümpel A, Chan AK, Diagnosis and management of neonatal purpura fulminansSemin Fetal Neonatal Med 2011 16(6):318-22. [Google Scholar]
[6]. Knoebl PN, Severe congenital protein C deficiency: the use of protein C concentrates (human) as replacement therapy for life-threatening blood-clotting complicationsBiol Targets Ther 2008 2(2):285-96. [Google Scholar]
[7]. Devi RU, Bharathi SM, Kawankar N, A novel protein C mutation causing neonatal purpura fulminansIndian Pediatr 2016 53(11):1019-21. [Google Scholar]
[8]. Monagle P, Chan AKC, Goldenberg NA, Ichord RN, Journeycake JM, Nowak-Gottl U, Antithrombotic therapy in neonates and children: Antithrombotic therapy and prevention of thrombosis, 9th American college of chest physicians evidence-based clinical practice guidelinesChest 2012 141(2 Suppl):e737S-801S. [Google Scholar]
[9]. Khor B, Van Cott EM, Laboratory tests for protein C deficiencyAm J Hematol 2010 85(6):440-42. [Google Scholar]
[10]. Reverdiau-Moalic P, Delahousse B, Body G, Bardos P, Leroy J, Gruel Y, Evolution of blood coagulation activators and inhibitors in the healthy human fetusBlood 1996 88(3):900-06. [Google Scholar]
[11]. Karpatkin M, Mannuccio Mannucci P, Bhogal M, Vigano S, Nardi M, Low protein C in the neonatal periodBr J Haematol 1986 62(1):137-42. [Google Scholar]
[12]. Williams MD, Chalmers EA, Gibson BE, The investigation and management of neonatal haemostasis and thrombosisBr J Haematol 2002 119(2):295-309. [Google Scholar]
[13]. Miletich J, Sherman L, Broze G, Absence of thrombosis in subjects with heterozygous protein C deficiencyN Engl J Med 1987 317(16):991-96. [Google Scholar]
[14]. Hu L, Zhou Y, Cao Y, Case Report A case of a new mutation of PROC causing neonatal purpura fulminansInt J ClinExp Med 2017 10(2):4005-07. [Google Scholar]
[15]. Kroiss S, Albisetti M, Use of human protein C concentrates in the treatment of patients with severe congenital protein C deficiencyBiol Targets Ther 2010 4:51-60. [Google Scholar]