Case Report
A 57-year-old male patient diagnosed with multiple myeloma presented to emergency with complaints of altered sensorium and progressive weakness of both upper and lower limbs for three days. The patient was on regular chemotherapeutic drugs including steroids, vincristine, adriamycin and melphalan. The investigation of patient revealed haemoglobin of 8.6 gm/dL, Total Leucocyte Count (TLC) of 12.24x103 UL, blood urea 32 mg/dL, serum creatinine 0.8 mg/dL, total bilirubin 0.2 mg/dL, alanine aminotransferase 38 U/L and aspartate aminotransferase 44 U/L. Neuroimaging of the brain showed multiple ill defined hyper dense lesions in left cortical and subcortical areas.
Due to high grade fever and focal neurological deficits, blood and CSF were sent for microbiological examination. The patient was empirically started on parenteral amikacin (1 gm/day) and ceftriaxone (1 gm/day)
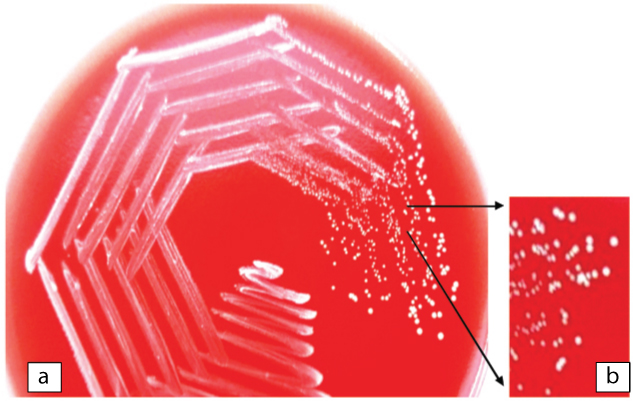
Blood and CSF specimens were processed using standard microbiological procedures. Gram stained smear of the CSF showed few pus cells with Gram positive cocci, bacilli and filaments. Based on Gram stain report the patient was started on metronidazole (500 mg TDS) and vancomycin (1 gm/day). CSF was inoculated on blood agar and MacConkey agar. After 48 hours of incubation at 37°C, blood agar showed 0.5 mm, dry, white opaque colonies [Table/Fig-1], and MacConkey agar showed tiny, dry, pale colonies. Nocardia spp. was suspected on basis of Gram stain of CSF which revealed Gram positive bacilli with filaments and growth on blood agar showing dry, opaque white colonies. Modified Ziehl-Neelsen staining was done on the smears from growth on the culture plate and the CSF which did not reveal any evidence of Nocardia. The blood sample, which was incubated in BactT/Alert 3D (Bio Me’rieux, Durham, North Carolina/USA), showed a positive signal after 72 hours of incubation. Gram stain from the broth showed Gram positive filaments. The broth was subcultured on blood agar and MacConkey agar. Growth similar to that from CSF was obtained on blood and MacConkey agar after 48 hours of aerobic incubation at 37°C. The growth on blood agar from CSF as well as blood culture was subjected to Gram staining which showed Gram positive bacilli with few filaments. A set of biochemical reactions were put to differentiate the Gram positive organisms having similar Gram stain picture [Table/Fig-2]. Gram positive rod and coccus forms can occur in Arthrobacter spp., Brevibacterium spp., Cellulomonas spp., Dermabacter spp., Oerskovia spp. and Rothia dentocariosa. Presence of fermentative metabolism, organism being non motile, white pigmented colonies, lactose non fermenter, nitrate reducer and no decarboxylation of lysine and ornithine favoured the identification of causative agent as Rothia spp. [1-3].
a) Dry, wrinkled, white colonies of Rothiadentocariosa on plate of blood agar; b) Magnified image of dry, wrinkled white colonies on blood agar.
Biochemical test results done for identification of the isolate.
| Biochemical test | Result |
|---|
| Catalase | Positive |
| Oxidase | Negative |
| Hanging drop | Non motile |
| Nitrate reduction | Positive |
| Triple sugar iron agar | A/A* |
| Urease | Negative |
| Sugar fermentation |
| Maltose | Fermented |
| Trehalose | Fermented |
| Glucose | Fermented |
| Fructose | Fermented |
| Sucrose | Fermented |
| Salicin | Fermented |
| Inositol | Not fermented |
| Lactose | Not fermented |
| Amino acid decarboxylation |
| Lysine | Not decarboxylated |
| Ornithine | Not decarboxylated |
*Triple sugar iron agar showing acid slant and acid butt.
Rothia mucilaginosa and Rothiaaeria are Gram positive cocci but Rothia dentocariosa shows Gram positive bacilli with rudimentary branching. Rothia mucilaginosa produces smooth mucoid colonies whereas Rothia dentocariosa produces white, dry, wrinkled colonies [Table/Fig-1] [2]; which further support the identification of isolate as Rothia dentocariosa.
Antibiotic susceptibility testing was done by Kirby-Bauer disc diffusion method on blood agar. We assessed the organism drug susceptibility as per 2014 Clinical and Laboratory Standards Institute (CLSI) guidelines for Staphylococcus (M 100-S 24) as no CLSI guideline is given for Rothia dentocariosa [4]. The isolate was found to be sensitive to tetracycline, ciprofloxacin, gentamicin, linezolid and vancomycin. It was resistant to erythromycin, clindamycin, cefoxitin and penicillin. The patient was on vancomycin and metronidazole, following our antibiotic sensitivity testing vancomycin was continued for one or more day. Condition of the patient deteriorated and he succumbed to the illness by the eighth day of hospitilsation.
Discussion
Rothia dentocariosa, a member of family Micrococcaceae, is a Gram positive organism found in the human oral cavity [5,6]. Rothia dentocariosa is a pleomorphic Gram positive bacterium that is seen as coccoid, rods and filamentous forms with possible branching [6,7]. They are non motile and non acid fast [7].
Roth in 1957 isolated the organism from dental plaque and caries; it was then called variously as Actinomyces dentocariosa, Nocardia dentocariosa or Nocardiasalivae. In 1967, detailed identification was done by Brown and genus Rothia was created [7]. In 1975, the first human infection (periappendiceal abscess) caused by Rothia dentocariosa was described [8]. On sheep blood agar after 24 hours of incubation at 37°C, colonies are non haemolytic, small, dry, white and adherent to media. On prolonged incubation the surface becomes rough with colonies showing a spoked wheel appearance, no aerial mycelia are seen [7].
Rothia can be differentiated from Nocardia as there is lack of acid fastness, absence of aerial mycelia and they ferment carbohydrates- features which are not seen in Nocardia. Actinomycetes grow anaerobically but Rothia spp. grows best aerobically which is used as the differentiating feature [5].
In a review article by Von Graevenitz A, fermentation of sugars such as fructose, glucose, maltose and sucrose by Rothia spp. was found to be 100% positive and that of ribose, salicin and trehalose was found to be more than 90% positive. Fermentation of arabinose, cellobiose, glycogen, inositol, mannose, starch and xylose was 100% negative; whereas lactose, mannitol, raffinose, rhamnose were 90% negative [3].
Rothia is known to cause infections such as bacteraemia, endocarditis, meningitis, peritonitis, bone and joint infection, pneumonia, skin and soft tissue infection, endophthalmitis and prosthetic device infection [9].
Rothia is known to cause infections especially in immune-compromised patients. Common source of bacteraemia among cancer patients are gut translocation, mucositis and catheter related infection. Risk factors for invasive disease are neutropenia and haematological malignancy, diabetes mellitus, chronic alcoholism, chronic liver disease and infection with HIV [9]. Our patient had multiple myeloma and was on steroids.
[Table/Fig-3,4] briefly outline the reports of Rothia dentocariosa isolated from various specimens among cancer patients and from blood across various parts of world respectively.
Rothia dentocariosa isolated from different specimens of cancer patients across various parts of the world.
| Reference | Underlying medical condition | Number of isolates | Complication | Specimen | Year and place of isolation | Treatment given and response to treatment |
|---|
| Schiff MJ et al., [11] | AML | 1 | Pneumonia | BAL; transthoracic aspirate | 1987; New York | Clindamycin; recovered |
| Pers C et al., [10] | CLL | 1 | Septicaemia | Blood | 1987; Denmark | Penicillin; expired |
| Wallet F et al., [12] | Lung cancer | 2 | Pneumonia | BAL | 1997; France | Amoxicillin clavulanic acid, Trimethoprim sulphamethoxazole; recovered |
| Kong R et al., [6] | Hepatic carcinoma | 1 | Endocarditis | Heart valves | 1998; France | Netilmycin, metronidazole and amoxicillin, recovered |
| Salamon SA and Prag J [5] | 1. Rectal cancer2. Tooth abscess3. Previous myocardial infarction, diverticulitis | 3 | Septicaemia | Blood | 2002; Denmark | 1. no antibiotics- expired2. and 3. penicillin G–recovered |
*AML- Acute Myeloid Leukaemia, BAL- Broncho Alveolar Lavage, CLL – Chronic Lymphocytic Leukaemia.
Rothia dentocariosa isolated from blood having central nervous system complications.
| Reference | Underlying medical condition | Number of isolates | Complication | Specimen | Year and place of isolation | Treatment given and response to treatment |
|---|
| Isaacson JH and Grenko RT [13] | Bicuspid aortic valve | 1 | Endocarditis, brain abscess | Blood | 1988; Vermont | Penicillin, gentamycin; recovered |
| Binder D et al., [14] | 1 mitral regurgitation, aortic insufficiency2. prosthetic aortic valve3. aortic composite graftAll 3 patients had periodontal disease | 3 | 1 Endocarditis and multiple brain abscess2. Endocarditis3. Endocarditis | Valve and blood | 1997; Switzerland | 1. Penicillin, netilmicin, vancomycin; expired2. Rifampicin, ciprofloxacin; recovered3. Rifampicin, ceftriaxone; recovered |
| Ricaurte JC et al., [8] | Root canal done | 1 | Endocarditis and multiple intracranial haemorrhage | Blood | 2001; New York | Vancomycin, gentamicin and penicillin G; recovered |
| Boudewijns M et al., [7] | treated for congenital bicuspid valve | 1 | Endocarditis, subarachnoid haemorrhage and intracranial aneurysm | Blood | 2003; Belgium | Penicillin and amikacin; recovered |
| Sadhu A et al., [15] | Mitral valve prolapse with MR, dental extractions | 1 | Multiple cerebellar haemorrhages | Blood | 2005; Arizona, USA | Penicillin G and gentamycin, recovered |
| Present study | Multiple myeloma | 1 | Brain abscess | Blood, cerebrospinal fluid | 2015; India | Vancomycin, expired |
Rothia dentocariosa has been isolated from both haematological and solid malignancy patients. Salamon SA et al., and Pers C et al., have reported isolation of Rothia from blood among cancer patients [5,10]. The antibiotic regimen for this organism has not been described. Treatment given to these oncology patients was clindamycin, penicillin, amoxicillin-clavulanic acid and trimethoprim-sulfamethoxazole [5,6,10-12]. In our patient, vancomycin was given as the strain was resistant to penicillin.
Isaacson JH et al., and Binder D et al., had shown brain abscess as a complication due to Rothia dentocariosa [13,14]. Ricaurte JC et al., reported a patient having endocarditis and multiple intracranial haemorrhages due to Rothia dentocariosa following a root canal treatment. The patient responded to treatment with vancomycin [8]. In 2003, Boudewijns M et al., reported a case of endocarditis, subarachnoid haemorrhage and intracranial aneurysm caused by Rothia dentocariosa and the patient recovered with penicillin and amikacin treatment [7]. Sadhu A et al., had discussed multiple cerebellar haemorrhages as a complication and the patient responded to penicillin and gentamicin treatment [15].
Conclusion
To the best of literature search, isolates of Rothia dentocariosa have not been reported among patients with haematological malignancy from India. Rothia dentocariosa is a low virulence organism, but is an emerging pathogen among immunocompromised patients. It is difficult to identify this organism and it can easily be mistaken for a contaminant. It is routinely not included in the database of automated systems. Hence, this organism is under-reported. Suspicion of this organism is important while treating immunocompromised patients with bacteraemia.
*Triple sugar iron agar showing acid slant and acid butt.
*AML- Acute Myeloid Leukaemia, BAL- Broncho Alveolar Lavage, CLL – Chronic Lymphocytic Leukaemia.
[1]. Forbes BA, Sahm DF, Weissfeld AS, Listeria, Corynebacterium and similar organisms. In: Forbes BA, Sahm DF, Weissfeld AS edBailey and Scott’s Diagnostic Microbiology 2007 12th edMissouriMosby Elsevier:287-97. [Google Scholar]
[2]. Procop GW, Church DL, Hall GS, Janda WM, Koneman EW, Schreckenberger PC, Koneman’s Color Atlas and Textbook of Diagnostic Microbiology 2017 7th edUSALippincott Williams and Wilkins:871-930. [Google Scholar]
[3]. Von Graevenitz A, Rothia dentocariosa: taxonomy and differential diagnosisClin Microbiol Infect 2004 10(5):399-402. [Google Scholar]
[4]. Verrall AJ, Robinson PC, Tan CE, Mackie WG, Blackmore TK, Rothia aeria as a cause of sepsis in a native jointJ of Clin Microbiol 2010 48(7):2648-50. [Google Scholar]
[5]. Salamon SA, Prag J, Three cases of Rothia dentocariosa bacteraemia: Frequency in Denmark and a reviewScand J Infect Dis 2002 34(2):153-57. [Google Scholar]
[6]. Kong R, Mebazaa A, Heitz B, De Briel DA, Kiredjian M, Raskine L, Case of triple endocarditis caused by Rothia dentocariosa and results of a survey in FranceJ Clin Microbiol 1998 36(1):309-10. [Google Scholar]
[7]. Boudewijns M, Magerman K, Verhaegen J, Debrock G, Peetermans WE, Donkersloot P, Rothia dentocariosa endocarditis and mycotic aneurysms: case report and review of literatureClin Microbiol Infect 2003 9(3):222-29. [Google Scholar]
[8]. Ricaurte JC, Klein O, LaBombardi V, Martinez V, Serpe A, Joy M, Rothia dentocariosa endocarditis complicated by multiple intracranial haemorrhageSouth Med J 2001 94(4):438-40. [Google Scholar]
[9]. Ramanan P, Barreto JN, Osmon DR, Tosh PK, Rothia bacteraemia: a 10 year experience at Mayo clinic, Rochester, MinnesotaJ Clin Microbiol 2014 52(9):3184-89. [Google Scholar]
[10]. Pers C, Kristiansen JE, Jonsson V, Hansen NE, Rothia dentocariosa in a patient with chronic lymphocytic leukaemia and toxic granulocytopeniaDan Med Bull 1987 34(6):322-23. [Google Scholar]
[11]. Schiff MJ, Kaplan MH, Rothia dentocariosa pneumonia in an immunocompromised patientLung 1987 165(5):279-82. [Google Scholar]
[12]. Wallet F, Perez T, Roussel-Delvallez M, Wallaert B, Courcol R, Rothia dentocariosa: two new cases of pneumonia revealing lung cancerScand J Infect Dis 1997 29(4):419-20. [Google Scholar]
[13]. Isaacson JH, Grenko RT, Rothia dentocariosa endocarditis complicated by brain abscessAm J Med 1988 84(2):352-54. [Google Scholar]
[14]. Binder D, Zbinden R, Widmer U, Opravil M, Krause M, Native and prosthetic valve endocarditis caused by Rothia dentocariosa: diagnostic and therapeutic considerationsInfection 1997 25(1):22-26. [Google Scholar]
[15]. Sadhu A, Loewenstein R, Klotz SA, Rothia dentocariosa endocarditis complicated by multiple cerebellar haemorrhagesDiagn Microbiol Infect Dis 2005 53(3):239-40. [Google Scholar]