Plasma Cell Leukaemia (PCL) is rare and an aggressive disease accounting for only 2-3% of all plasma cell dyscrasias. Diagnosis is made when there are more than 2x109/L plasma cells in the peripheral blood or monoclonal plasmacytosis more than 20% of the Total Leucocyte Count (TLC). We report a case of 58-year-old male with history of high grade fever, generalized weakness and giddiness for two to three months. Peripheral blood film revealed marked leucocytosis with 88% of atypical cells resembling blasts. Flow cytometric immunophenotyping confirmed plasmacytic lineage. Bone marrow was planned but the patient deteriorated very rapidly and died within 3 days. PCL has no definitive treatment and has a dismal prognosis, requiring more extensive data to improve the disease course.
Case Report
A 58-year-old male patient was admitted to our hospital with complaints of continuous fever, generalized weakness, chest pain and giddiness for two months. He was a smoker for 15 years. On clinical examination severe anaemia, hepatosplenomegaly and bleeding from nose was present. He showed tachycardia with pulse rate 116/min, blood pressure 140/75 mmHg and temperature 1020F. The complete blood counts, biochemical investigations, peripheral smear examination and flowcytometric immunophenotyping were done [Table/Fig-1]. The bone marrow procedure was planned, but the patient’s condition deteriorated very rapidly and he expired before it could be done.
Clinical and Immunophenotypic data of the patient.
| S.NO | Parameter | Present Case |
|---|
| 1. | Haemoglobin (Hb) gm/dL | 7.2 (13-17) |
| 2. | Total Leucocyte Count (TLC/mm3) | 1,20,000 (4,000-11,000) |
| 3. | Platelet (PLT) /mm3) | 30,000 (1,50,000-4,50,000) |
| 4. | Peripheral blood plasma cell count (%) | 88 |
| 5. | Serum LDH (240-480U/L) | 546 (240-480) |
| 6. | Serum creatinine (mg%) | 1.7 (0.7-1.2) |
| 7. | Type of monoclonal protein | IgG |
| 8. | Immunophenotype | Positive markers = CD38, CD138, CD117,Negative markers = CD19, CD20, CD45, CD56, CD1a, CD2, CD3, CD4, CD7, CD8, CD13, CD33, CD14, CD15, CD22, CD34,HLADR, CD61,CD64,GlycophorinA,cytoCD3,cytoCD79a,MPO. |
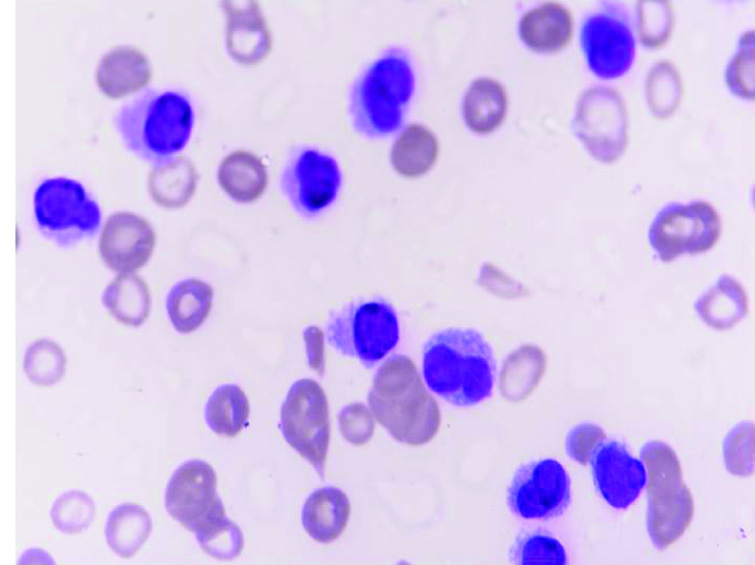
Serum calcium, blood urea, other electrolytes and liver function tests were normal. The peripheral smear showed 88% atypical blast like cells, neutrophils 3% and lymphocytes 9%. The atypical cells were large cells with central and cleaved nuclei, opened up chromatin, scant to moderate lightly basophilic cytoplasm, few with prominent nucleoli and cytoplasmic projections [Table/Fig-2]. A diagnosis of acute leukaemia was made.
Peripheral smear showing atypical cells with cleaved nuclei, clumped chromatin, prominent nucleoli, moderate basophilic cytoplasm and cytoplasmic projections (Giemsa Stain; 100X).
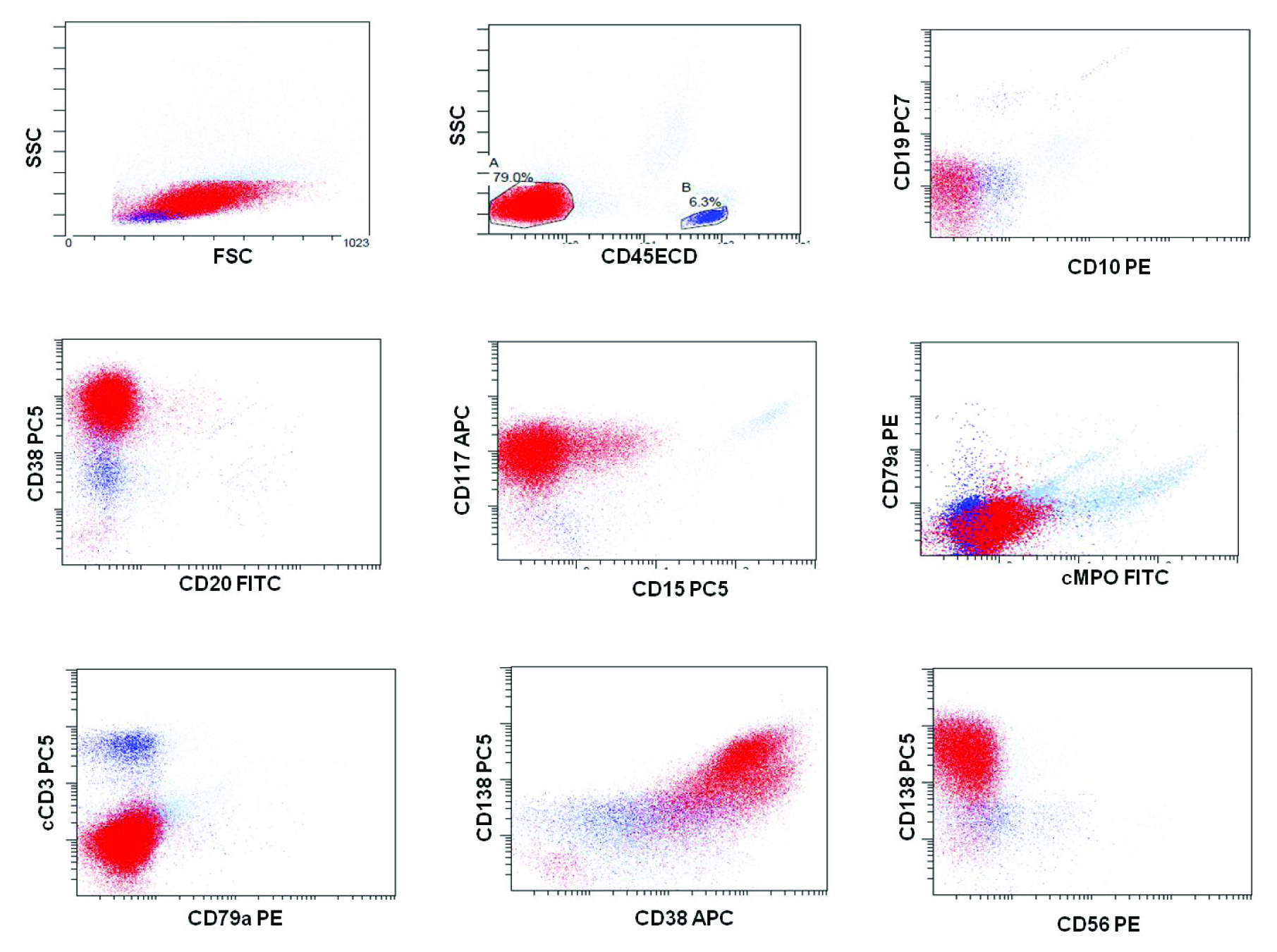
Flowcytometric Immunophenotyping was performed on peripheral blood using six colour flow cytometry Navios (Beckman Coulter) using standard staining and analytic methods. The total events counted were 100,000. The acute leukaemia panel of antibodies to leukocyte-associated markers were run which included surface CD1a, CD2, CD3, CD4, CD5, CD7, CD8,CD10, CD13, CD14, CD15, CD19, CD20,CD22, CD33, CD34, CD38, HLA-DR, CD45, CD56, CD61, CD64, Glycophorin A, CD138, CD117, cytoplasmic CD3, cytoplasmic CD79a, Myeloperoxidase (MPO), Terminal deoxynucleotidyl Transferase (TdT). Selected antibody combinations were conjugated to Fluorescein Isothiocyanate (FITC), phycoerythrin (PE), phycoerythrin-Texas Red conjugate (ECD), PE-Cyanine 5 (PC5), PE-Cyanine 7 (PC7) and Allophycocyanin (APC). The atypical cells were gated using CD45 versus side scatter (SSC) strategy and showed a single population of cells negative for CD45 with low side scatter and constituted 70% of the total cells. The cells were bright positive for CD38 and dim positive for CD117. All other markers for Acute leukaemia were negative. Due to bright CD38 positivity and negative CD45, a plasma cell leukaemia was suspected and markers CD138, CD56 were added. The atypical cells were bright positive for CD138 and negative for CD56 [Table/Fig-3]. A sample for Serum Protein Electrophoresis (SPEP) and Immunofixation (IFE) was obtained which showed a M-band in gamma region and corresponded to IgG Kappa on immunofixation. Based on clinical features, SPEP/IFE and flowcytometric immunophenotyping findings on peripheral blood, a diagnosis of primary Plasma Cell Leukaemia (pPCL) was established. The patient’s condition deteriorated very rapidly and expired in less than 72 hours of hospitalization; so, no specific treatment could be offered.
On dot plots; the atypical cells (79%) were bright positive for CD38, CD138 and dim positive for CD117. These cells were negative for other markers such as CD45, cMPO, CD19, CD20, CD10, CD15, CD79a, cCD3 and CD56.
Discussion
PCL is a rare plasma cell neoplasm characterized by circulating clonal plasma cells more than 2x109/L or plasma cells constituting at least 20% of the differential leukocyte count. It is classified as primary or de novo (primary PCL) and secondary after leukaemic phase of pre-existing, long standing multiple myeloma (secondary PCL). pPCL constitutes 1.3-3.4% of all plasma cell dyscrasias [1]. The clinical presentation includes non-specific symptoms like fever, fatigue, weight loss, bony pains and organomegaly [1].
pPCL has some common manifestations of acute leukaemia such as leucocytosis with high Nuclear-Cytoplasmic (N/C) ratio, anaemia, thrombocytopenia and organomegaly [2]. Our patient, presented as leukaemia having features of fever, generalized weakness, hepatosplenomegaly, marked leucocytosis with severe anaemia and thrombocytopenia. The Lactate Dehydrogenase (LDH) levels were high 546U/L (240-480U/L). A higher proportion of patients with pPCL have significant leucocytosis, elevated LDH and serum β2-microglobulin levels reflecting its clinical behaviour [3]. Anaemia with haemoglobin level less than 9 gm/dl occurs in 80% of primary PCL versus 35% of cases of Multiple Myeloma (MM). Thrombocytopenia with platelet count less than 100X109/L is seen more commonly in pPCL than in MM [4]. Our patient presented with severe anaemia and thrombocytopenia. The patient had massive hepatosplenomegaly, which is more common in pPCL. There was absence of lytic bone lesions on X-ray. Lytic bone lesions are more commonly seen in secondary plasma cell leukaemia [5]. An elevated Blood Urea Nitrogen (BUN) and serum creatnine is seen in 75% cases of pPCL verses 40% cases of multiple myeloma; as was seen in our patient [4].
The correct diagnosis of PCL relies upon the ability of the pathologist to screen and recognize plasma cells in the peripheral blood smear [3]. However, in our case the peripheral blood smear showed 88% of atypical cells resembling blasts. The atypical cells were large cells with central and cleaved nucleus, opened up chromatin, scant to moderate lightly basophilic cytoplasm, few with prominent nucleoli and cytoplasmic projections. The typical plasmacytoid morphology was not seen and therefore, acute leukaemia was suspected.
Flowcytometric immunophenotyping is an important diagnostic tool for evaluation and confirmation of circulating clonal plasma cells. Normal plasma cells are CD19, CD20, CD22, CD27, CD38 and CD138 posititve, however, the clonal plasma cells also express CD38 and CD138 with absence of normal plasma cell marker like CD19 and CD45+/- [6]. There is reduced expression of CD117, HLA-DR and CD9 in comparison to multiple myeloma. Higher expression of CD20 is seen in pPCL [7].
In our case, these cells expressed bright CD38, CD138 and dim CD117 on flow cytometric immunophenotyping and were confirmed to be of plasmacytic lineage. These cells were negative for CD45, CD34, CD20, CD56 and other myeloid and lymphoid markers, ruling out acute leukaemia and confirming the diagnosis of primary PCL. Lack of CD56 is a hallmark of pPCL and is a bad prognostic marker [8]. In previous literature, extensive bone marrow infiltration, often with plasmablastic cytomorphology is more commonly reported in pPCL than sPCL [9]. Bone marrow examination was planned in our patient also, but his condition deteriorated very rapidly and the patient expired in 72 hours, before bone marrow could be performed. Comprehensive immunophenotyping is instrumental for accurate diagnosis of plasma cell leukaemia mimicking acute leukaemia.
Cytogenetic analysis by Fluorescent In Situ Hybridization (FISH) can be performed on bone marrow aspirate. The most common cytogenetic abnormality seen in pPCL is t (11;14). Other abnormalities include del (17p13), del (13q), del (1p21) and other chromosome 14 abnormalities [6].
There are many controversies regarding the treatment of PCL. Alkylating agents in conventional doses have not shown any significant activity. Although, higher response rates have been reported with use of VAD like regimes, overall outcome of patients has not changed [9]. Regimens incorporating hyperfractionated cyclophosphamide, such as Hyper-CVAD, have demonstrated limited success in PCL [10]. Recently, thalidomide, bortezomib and lenalidomide have been tried in PCL. Bortezomib used as single agent or in combination has been reported to improve overall remission rate [11]. However, now a days, stem cell transplant is being tried in pPCL which showed a significant improvement in overall survival following Hematopoietic Stem Cell Transplantation (HSCT) [12]. But lack of effective induction regime and rapid decline in patient performance status has precluded HSCT as a viable option [13]. Plasma cell leukaemia remains an incurable and highly resistant disease with extremely poor prognosis. Primary PCL may respond to treatment initially, but these responses are short lived with median survival of eight months. Secondary PCL is extremely resistant, more rapidly progressive and fatal disease with median overall survival of two months [14]. Our patient’s condition was very serious and he deteriorated very rapidly and expired in less than 72 hours of hospitalization; so, no specific treatment could be offered. In conclusion, patients with pPCL have an aggressive clinical presentation and poor prognosis; subsequent from a different biological background compared with classic multiple myeloma. Survival of most pPCL patients is still inferior compared with outcome in newly diagnosed multiple myeloma, indicating the need for novel treatment strategies [15].
Conclusion
In summary, we describe a case of primary PCL with very short unfavorable outcome which illustrates the importance to know this rare and fatal disease which can mimic acute leukaemia and can be diagnosed only by comprehensive immunophenotyping. Haematologists and pathologists should be aware of the clinical relevance of a careful morphological examination of peripheral blood smears to exclude the presence of circulating plasma cells and plasmablasts.
[1]. Ramsingh G, Mehan P, Luo J, Vij R, Morgensztern D, Primary plasma cell leukaemia: a surveillance, epidemiology, and end results database analysis between 1973 and 2004Cancer 2009 115(24):5734-39. [Google Scholar]
[2]. Colovic M, Jankovic G, Suvajdzic N, Milic N, Dordevic V, Jankovic S, Thirty patients with primary plasma cell leukaemia: a single center experienceMed Oncol 2008 25(2):154-60. [Google Scholar]
[3]. Fernández de Larrea C, Kyle RA, Durie BG, Ludwig H, Usmani S, Vesole DH, Plasma cell leukaemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working GroupLeukaemia 2013 27(4):780-91. [Google Scholar]
[4]. Kosmo MA, Gale RP, Plasma cell leukaemiaSemin Hematol 1987 24(3):202-08. [Google Scholar]
[5]. Gupta A, Gupta C, Kapoor A, An unusual presentation of primary plasma cell leukaemia as a psychiatric disorder: a diagnostic dilemma- a case report and review of literatureIndian Journal of Pathology and Oncology 2015 2(1):48-52. [Google Scholar]
[6]. Gonsalves WI, Primary plasma cell leukaemia: A practical approach to diagnosis and clinical managementAJHO 2017 13(3):21-25. [Google Scholar]
[7]. Perez-Andres M, Almeida J, Martin-Ayuso M, Moro MJ, Martin-Nunez G, Galende J, Clonal plasma cells from monoclonal gammopathy of undetermined significance, multiple myeloma and plasma cell leukaemia show different expression profiles of molecules involved in the interaction with the immunological bone marrow microenvironmentLeukaemia 2005 19(3):449-55. [Google Scholar]
[8]. Pellat-Deceunynck C, Barillé S, Jego G, Puthier D, Robillard N, Pineau D, The absence of CD56 (NCAM) on malignant plasma cells is a hallmark of plasma cell leukaemia and of a special subset of multiple myelomaLeukaemia 1998 12(12):1977-82. [Google Scholar]
[9]. Costello R, Sainty D, Bouabdallah R, Fermand JP, Delmer A, Divine M, Primary plasma cell leukaemias: a report of 18 casesLeuk Res 2001 25(2):103-07. [Google Scholar]
[10]. Murthy V, Mwirigi A, Ward S, Rassam SMB, Rapid and excellent response to hyper CVAD, particularly with thalidomide, in plasma cell leukaemia and long term remissions following allogeneic stem cell transplantationASH Annual Meeting Abstracts 2009 114(22):4963 [Google Scholar]
[11]. Musto P, Rossini F, Gay F, Pitini V, Guglielmelli T, D’Arena G, Efficacy and safety of bortezomib in patients with plasma cell leukaemiaCancer 2007 109(11):2285-90. [Google Scholar]
[12]. Drake MB, Iacobelli S, van Biezen A, Morris C, Apperley JF, Niederwieser D, Primary plasma cell leukaemia and autologous stem cell transplantationHaematologica 2010 95(5):804-09. [Google Scholar]
[13]. Sher T, Miller KC, Deeb G, Lee K, Chanan Khan A, Plasma cell leukaemia and other aggressive plasma cell malignanciesBr J Haematol 2010 150(4):418-27. [Google Scholar]
[14]. Garcia-Sanz R, Orfao A, Gonzalez M, Tabernero MD, Blade J, Moro MJ, Primary plasma cell leukaemia: clinical, immunophenotypic, DNA ploidy, and cytogenetic characteristicsBlood 1999 93(3):1032-37. [Google Scholar]
[15]. Van de Donk NWCJ, Lokhorst HM, Anderson KC, Richardson PG, How I treat plasma cell leukaemiaBlood 2012 120(12):2376-89. [Google Scholar]