The present day concept of tumour immune surveillance was described earlier by Willis RA, when he proposed the idea that a tumour may modulate host reaction by producing an inflammatory response that may be protective against the tumour [1]. Although the association between lymphocytic infiltrate and tumour cell infiltration has been recognised for more than 100 years now [2,3], there are many types of cancers that are associated with increased eosinophilic infiltrate either within the tumour or in the blood or both. Presence of eosinophils as a component of peri and intratumoral inflammatory infiltrate is known as Tumour Associated Tissue Eosinophilia (TATE).
It has been observed that most of the tumours that show TATE are located at a body surface i.e mucosa or skin [4]. Leighton SEJ et al., characterised TATE as tumoral infiltration by eosinophils which cannot be attributed to the presence of necrosis and/or ulceration [5].
The association between TATE and the natural course of the tumour has been variably described as favourable, detrimental or having no influence on prognosis at all. In many studies, the favourable prognostic effects of TATE have been attributed to the presence of various cationic proteins in the eosinophilic granules like Major Basic Proteins (MBP), eosinophilic cationic protein, eosinophil-derived neurotoxin and eosinophil peroxidase along with the presence of other inflammatory mediators like GM-CSF, IL-3, IL-5, TNF-a, TGF-b. Both these cationic proteins as well as inflammatory mediators are considered to be related to host-cell (normal as well as tumour cells) lysis and hence considered responsible for the favourable prognostic effects of TATE [6]. However lately, it has been suggested that eosinophils have a role in tissue remodelling rather than having direct participation in tumour cell clearance [7]. The various cationic proteins, inflammatory mediators, proteases and growth factors that eosinophils synthesise are associated with both tissue angiogenesis and tissue remodelling. Some of these factors are transforming growth factor-α and β, [8] vascular endothelial growth factor [9] and matrix metalloprotease-9 [10]. Therefore, we can safely suggest that the role of eosinophils in tumour biology may be less due to direct tumoricidal activity and more towards modulation of tumour microenvironment.
The aim of our study was to study the association of TATE and metastases in squamous cell carcinomas irrespective of their site of origin. The association between TATE and microscopic morphological characteristics, as well as tumour-tissue inflammatory response was also studied.
Materials and Methods
This was a medical record based observational study conducted over a period of two years (June 2014 to June 2016). Institutional ethical clearance was obtained. A total of 148 cases of squamous cell carcinomas from various sites were included in the study.
Surgically resected and biopsy specimens of patients with squamous cell carcinomas, not previously subjected to treatment or who had undergone surgery as the initial treatment were included. Those with other simultaneous tumours, those undergoing radiotherapy/ chemotherapy and tumours with extensive necrosis and ulceration were excluded. Metastasis was considered as present if the histopathology sample received had involved lymph nodes; or if regional and/or distant metastasis was proven by any other cyto-pathological or radiological means as per the patients’ records.
All the surgically resected specimens/biopsies were processed routinely, cut into 4μ thin sections and stained with Haematoxylin and Eosin (H&E). A two tumour scale (Broders and Bauers) were used according to a study by Goldsmith MM et al. [11]. According to Broders scale, tumours were graded as well, moderately or poorly-differentiated based on the increasing percentage of undifferentiated epithelium. Using Bauer’s scale, tumours were designated as keratinizing or non keratinizing.
Grading of TATE was done from 0 to 4+ according to the criterion by Leighton SEJ et al. [5]. Grade ‘0’ was given to 0-2 eosinophils/High Power Field (HPF), ‘1+’ to 2-10 eosinophils/HPF, ‘2+’ to 10-20 eosinophils/HPF, ‘3+’ to 20-30 eosinophils/HPF and 4+ to >30 eosinophils/HPF. A total of 10 high power fields were assessed, the average number of eosinophils/HPF was taken and thereby the grade of TATE was assigned. Grades 0 to 2+ were taken as low grade tissue eosinophilia (TATE) while grades 3+ and 4+ as high grade TATE.
Pleomorphism was graded as grades 1 and 2 (low), 3 (moderate) and 4 (high) on observing 10%, 30%, 50% and 75% pleomorphic tumour cells respectively.
Desmoplasia was subjectively graded as minimal/low grade and high grade on the basis of increasing connective tissue and fibrosis around the tumour.
Inflammatory infiltrate was subjectively graded as sparse, moderate or intense based on the presence of neutrophils, lymphocytes or mast cells.
Pleomorphism, desmoplasia and inflammatory infiltrate were analysed in relation to the grade of TATE. Tumours were also studied in relation to pattern of tumour spread at the periphery (borders) i.e either pushing or infiltrating.
Results
Cervical cancers constituted most of our cases as seen in [Table/Fig-1]. Overall males 86 (58.1%) and females presented in a ratio of 1.38:1. Maximum number of patients (n=46) were in their fifth decade of life.
Distribution of cases of squamous cell carcinomas according to the site of origin.
| Sites of squamous cell carcinoma | Number (n=148) | Percentage |
|---|
| Cervix | 28 | 18.9 |
| Tongue | 18 | 12.16 |
| Oral cavity | 14 | 9.45 |
| Penis | 12 | 8.1 |
| Oesophagus | 12 | 8.1 |
| Skin Ulcers | 10 | 6.75 |
| Supraglottis | 10 | 6.75 |
| Face and scalp | 8 | 5.4 |
| Tonsil | 11 | 7.43 |
| Aryepiglottic | 4 | 2.7 |
| Glottis | 8 | 5.4 |
| Nose | 4 | 2.7 |
| Salivary Gland | 2 | 1.35 |
| Middle ear | 2 | 1.35 |
| Ethmoid sinus | 2 | 1.35 |
| Conjuctiva | 2 | 1.35 |
| Anal canal | 1 | 0.67 |
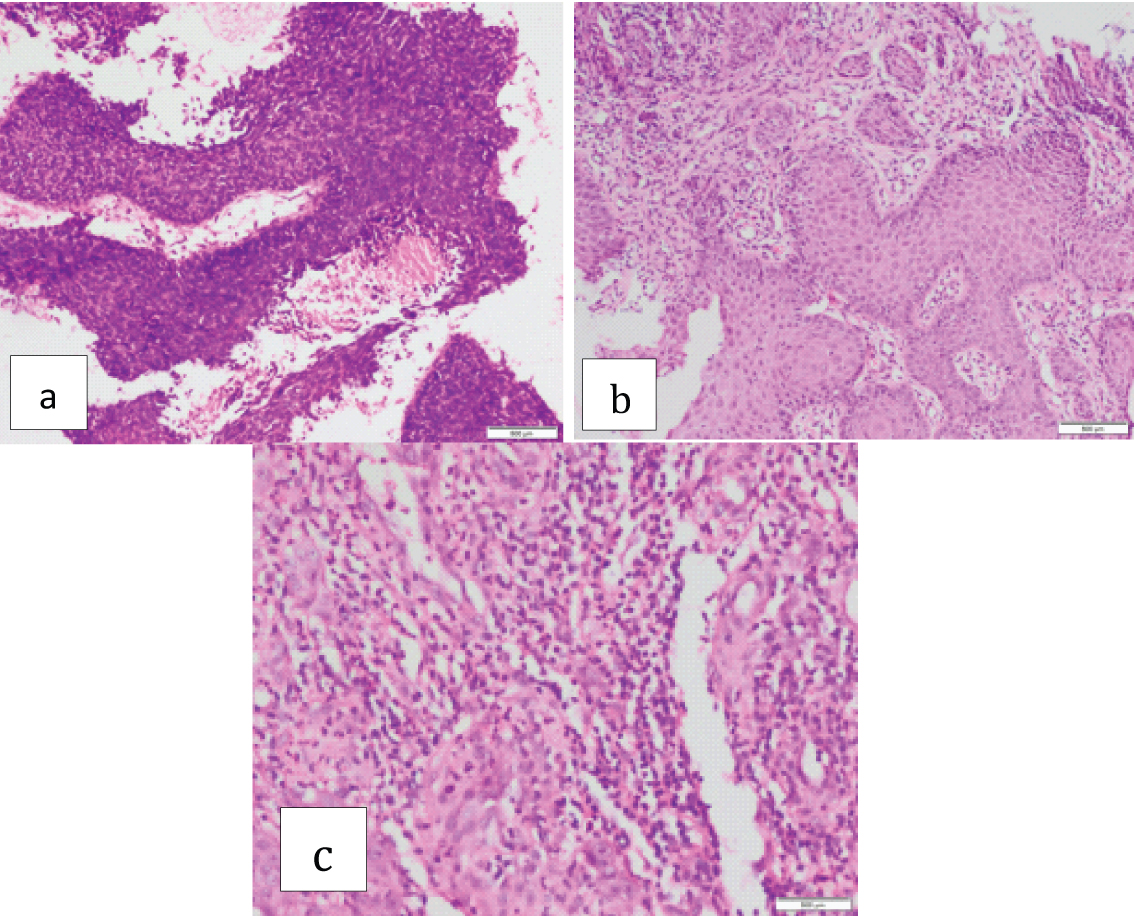
Tumours were well, moderately and poorly differentiated in 51 (34.4%), 82 (55.4%) and 15 (10.1%) cases respectively. The borders were either infiltrating 83 (56%) or pushing 65 (44%). The inflammatory infiltrate was sparse, moderate and intense in 21 (14.1%), 73 (49.3%) and 54 (36.4%) cases respectively [Table/Fig-2].
Squamous cell carcinoma with a) Sparse inflammatory infiltrate (1+) (H&E stain;10X); b) Moderate inflammatory infiltrate (2+) (H&E stain;40X); c) Intense inflammatory infiltrate (3+) (H&E stain;20X).
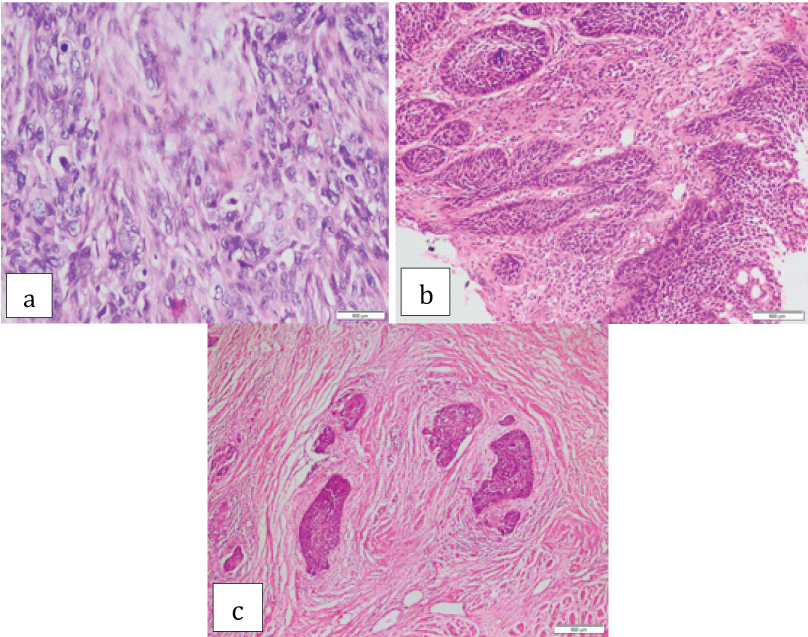
Low grade and high grade desmoplasia was observed in 54 (36.4%) and 94 (63.5%) cases respectively [Table/Fig-3].
Squamous cell carcinoma associated with a) Minimal/low grade desmoplasia (H&E 40X); b) Low grade desmoplasia (H&E 20X); c) High grade desmoplasia (H&E 40X).
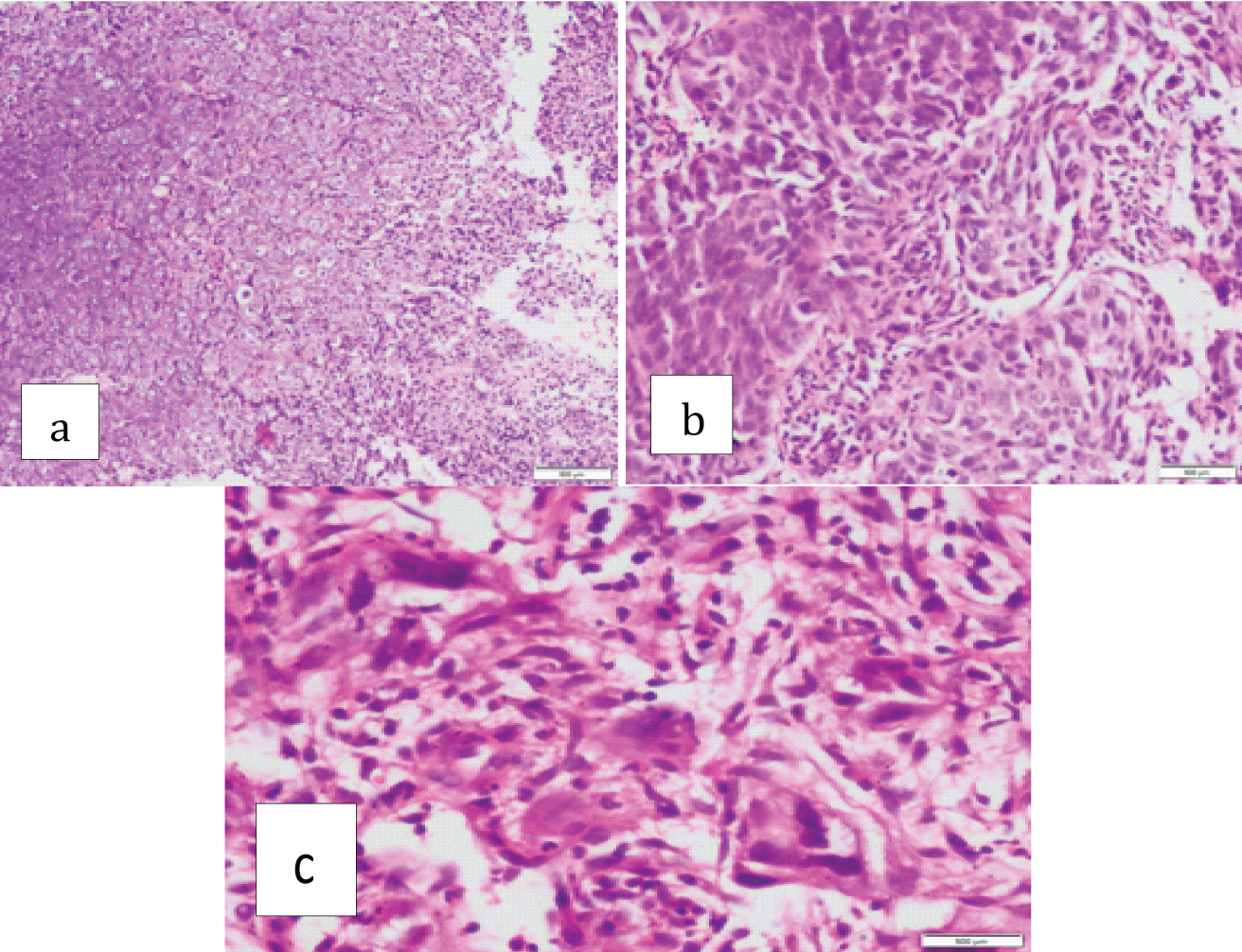
Grades 1+, 2+, 3+ and 4+ pleomorphism was observed in 54 (36.4%), 40 (27.0%), 28 (18.9%) and 26 (17.5%) cases respectively [Table/Fig-4].
Squamous cell carcinoma with associated with: a) Low pleomor-phism (1+/2+). (H&E stain;10X); b) Moderate pleomorphism (3+) (H&E stain;40X); c) High pleomorphism (4+) (H&E stain;40X).
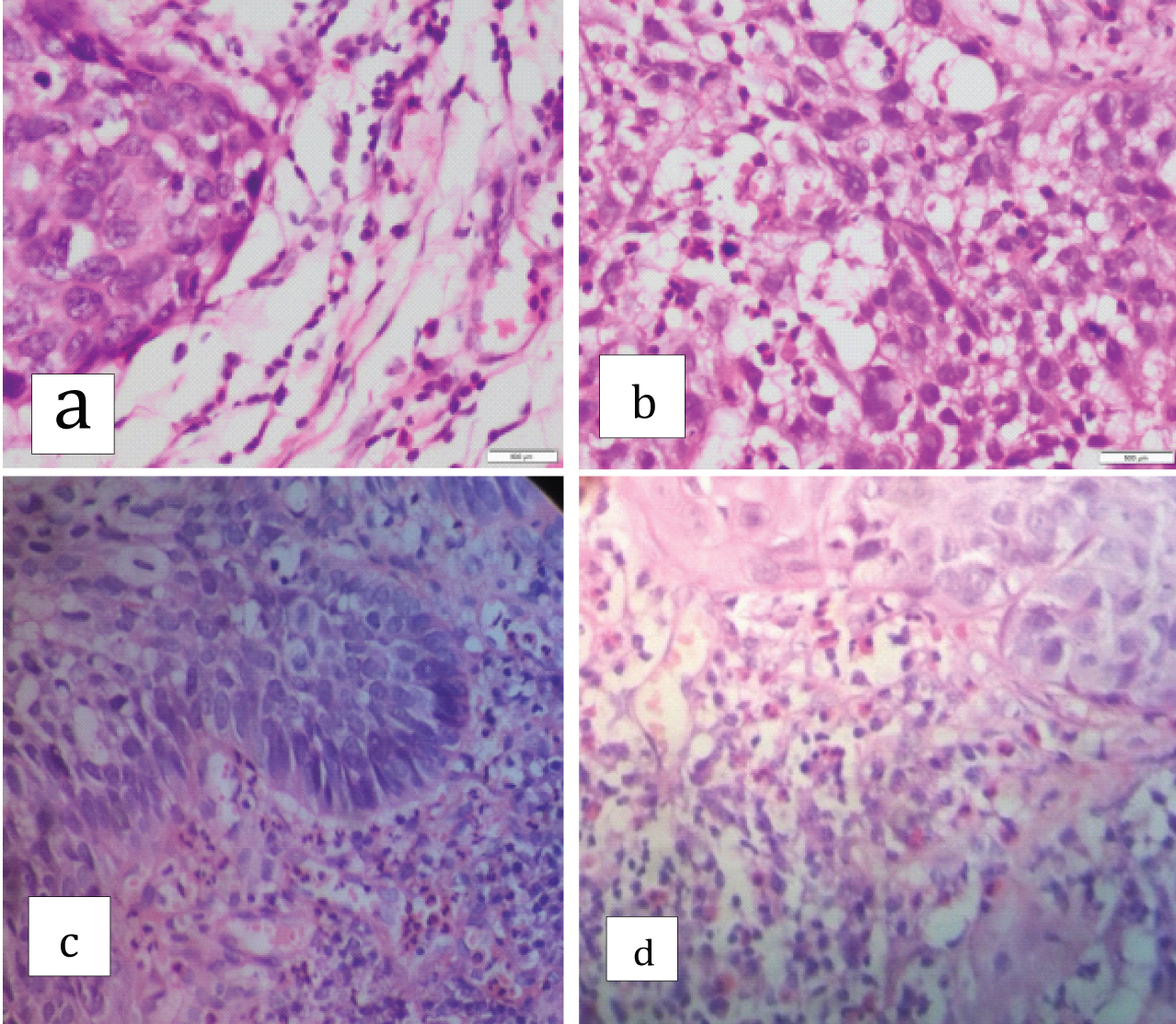
Grades 0, 1+, 2+, 3+ and 4+ TATE was observed in 20 (13.5%), 37 (25%), 40 (27.0%), 30 (20.2%) and 21 (14.1%) respectively [Table/Fig-5].
Well differentiated squamous cell carcinoma associated with: a) Low grade of tissue eosinophilia (TATE 0/ 1+); b) Low tissue eosinophilia (TATE 2+); c) High grade tissue eosinophilia (TATE 3+); d) High grade tissue eosinophilia (TATE 4+) (H&E stain;40X).
[Table/Fig-6] shows the association between grades of TATE and the histomorphological features.
Correlation of TATE with other histomorphological variables.
| Parameters | TATE (Grade 0,I,II)No. of cases (%)n=97 | TATE (Grade III-IV) No. of cases (%) n=51 | p-value |
|---|
| Grade of differentiation |
| Well-Differentiated | 20 (20.6) | 31 (60.8) | <0.001 |
| Moderately-Differentiated | 65 (67) | 17 (33.3) |
| Poorly Differentiated | 12 (12.3) | 3 (5.9) |
| Borders |
| Pushing | 23 (23.7) | 42 (82.3) | <0.001 |
| Infiltrating | 74 (76.2) | 9 (17.6) |
| Inflammatory infiltrate |
| Sparse | 17 (17.5) | 4 (7.8) | <0.001 |
| Moderate | 58 (59.7) | 15 (29.4) |
| Intense | 22 (22.6) | 32 (62.7) |
| Desmoplasia |
| Low grade | 50 (51.5) | 4 (7.8) | <0.001 |
| High grade | 47 (48.4) | 47 (92.1) |
| Pleomorphism |
| Low-Moderate | 55 (56.7) | 39 (76.4) | 0.0175 |
| High | 42 (43.2) | 12 (23.5) |
Tumour was metastasized in 45.9% cases (n=68). The sites of metastasis varied according to the primary site of malignancy; lymph nodes (94.1%), lungs (22.0%), liver (20.5%), peritoneum (5.8%), and so on, in isolation or in combination. The association between metastasis and TATE and histomorphological features is shown in [Table/Fig-7]. Metastasis was significantly associated (p<0.05) with low grades of TATE, infiltrating borders, intense inflammatory infiltrate and high grade pleomorphism.
TATE and other histomorphological variables with metastasis in squamous cell carcinomas.
| Parameters | Metastasis | p-value |
|---|
| Present (n=68) | Absent (n=80) |
|---|
| TATE |
| Low grade | 61 (89.7) | 36 (45) | <0.001 |
| High grade | 7 (10.2) | 44 (55) |
| BORDERS |
| Pushing | 21 (30.9) | 44 (55) | 0.003 |
| Infiltrating | 47 (69.1) | 36 (45) |
| INFLAMMATORY INFILTRATE |
| Sparse | 4 (5.8) | 17 (21.2) | <0.001 |
| Moderate | 25 (36.7) | 48 (60) |
| Intense | 39 (57.3) | 15 (18.8) |
| DESMOPLASIA |
| Low (1+/2+) | 29 (42.6) | 25 (31.2) | 0.151 |
| High (3+/4+) | 39 (57.3) | 55 (68.8) |
| PLEOMORPHISM |
| Low (1+/2+) | 13 (19.1) | 56 (70) | <0.001 |
| Moderate/High (3+/4+) | 55 (80.8) | 24 (30) |
Discussion
In the present study, low grades of TATE were associated with high degree of pleomorphism, low grade of desmoplasia, infiltrating borders, moderate inflammatory infiltrate and a tendency to spread. Conversely, high grade tissue eosinophilia was associated with low-grade pleomorphism, variable to high-grade desmoplasia, pushing borders, moderate to intense inflammatory infiltrate and low tendency to metastasize.
Following the original identification of TATE, it has also been described in cancers of cervix, oral cavity, gastrointestinal adenocarcinoma, transitional cell carcinoma of the bladder and head and neck tumours generally as well as in laryngeal and nasopharyngeal carcinomas specifically [12]. The number of studies that suggest that tumour tissue eosinophilia is associated with an improved prognosis are countered by an almost equal number of studies showing either no role of eosinophils or an association with a poor prognosis [Table/Fig-8].
Prognostic implications of TATE in various studies.
| Authors | Year* | Cases Studied | Site | Prognostic implications |
|---|
| Leighton SEJ et al. [5] | 1996 | 96 | Nasopharynx | None |
| Dorta RG et al. [6] | 2002 | 125 | Oral Cavity | Favourable |
| Sassler AM et al. [13] | 1996 | 284 | Larynx | No correlation between TATE and prognosis |
| Ohashi Y et al. [14] | 2000 | 35 | Oesophagus | No correlation |
| Wong DTW et al. [15] | 1999 | 20 | Oropharynx | Unfavourable |
| Debta P et al. [16] | 2011 | 30 | Oropharynx | Favourable |
| Oliveira DT et al. [17] | 2012 | 71 | Oropharynx | No correlation |
| Sahni P et al. [18] | 2015 | 30 | Oropharynx | Favourable |
| Inoue T et al. [19] | 1981 | 778 | Cervix | Favourable with LCNK (Large-Cell Non-Keratinizing) SCC |
| Goldsmith MM et al. [20] | 1987 | 82 | Head and Neck | Favourable |
| Present study | 2016 | 148 | Variable | Favourable |
* For the present study year in which the study was conducted has been mentioned. Other studies mention the year in which they were published.
The infiltration of inflammatory cells in cancer tissues is suspected to contribute to tumour growth and is considered a crucial aspect of host response in malignancies. There are many aspects of cancer phenotype namely proliferation, de-differentiation and tissue re-organization. All these may initiate and maintain influx of inflammatory cells both within as well as around the tumour. Studying specifically the degree of tumour tissue infiltration by eosinophilis, authors have found intense TATE in head and neck tumours which range from > 10 up to 100 eosinophils per HPF [5,12,13].
Relying solely on biopsy based studies to document the occurrence of TATE might not be extraplolated to squamous cell carcinomas across the board [5,13,14]. Since eosinophils are seen predominantly on the tumour invasion front and biopsy specimens which are small and superficial may not represent the actual quantum of tissue eosinophilia. Similarly exclusively counting the stromal infiltrating cells may also not be the true representative of tissue eosinophilia. It has been supported by the fact that both stromal and tumour eosinophilic infiltration was seen in 46.5% of the samples [6]. It is difficult to compare results obtained from different authors due to lack of information in these studies on relevant data such as the number of microscopic fields or the total area analysed [5,11,15-20].
Overall peritumoral inflammatory cell infiltration is considered a prognostic variable in solid tumours but the impact of individual cell type on survival has still not been evaluated fully and compared with the conventional disease classification. To detect the presence of intact and degranulating peritumoral eosinophilic infiltrate, various special techniques like autofluorescence or immunohistochemistry can also be used [6].
Most of the previously published studies have stressed on the importance of lymphocytes particularly T-lymphocytes as specific inflammatory cells inducing anti-cancer reaction [21]; however non-specific inflammatory reaction also have an important role with the presence of NK cells [22], macrophages [23], mast cells [24], neutrophils and eosinophils [25] having prognostic values in various solid cancers. The importance of different cells is usually analysed for a single cell type, but an integrated study of different cell types is required considering the complex interactions between the specific and general immunological reactions.
Conclusion
This study highlights the importance of tissue eosinophilia in the biologic behaviour of squamous cell carcinomas. Although, not proven, TATE has the potential to be used as a surrogate marker of metastasis in resource poor countries with limited access and affordability to advanced radiological techniques for tumour staging and metastasis.
Further research recruiting larger samples and from different sites are required to validate the results of the present study as well as to standardise the technique of assessing TATE to reduce intra and inter observer variability. The role of eosinophils as an important part of host surveillance mechanism against tumours needs to be evaluated further before it can be put into practical application.
* For the present study year in which the study was conducted has been mentioned. Other studies mention the year in which they were published.